Abstract
We systematically reviewed the effectiveness and safety of continuous subcutaneous insulin infusion (CSII) with insulin analogs compared with multiple daily injections (MDI) in pregnant women with diabetes mellitus. We searched Medline®, Embase®, and the Cochrane Central Register of Controlled Trials through May 2013. Studies comparing CSII with MDI in pregnant women with diabetes mellitus were included. Studies using regular insulin CSII were excluded. We conducted meta-analyses where there were two or more comparable studies based on the type of insulin used in the MDI arm. Seven cohort studies of pregnant women with type 1 diabetes reported improvement in hemoglobin A1c (HbA1c) in both groups. Meta-analysis showed no difference in maternal and fetal outcomes for CSII versus MDI. Results were similar when CSII was compared with MDI with insulin analogs or regular insulin. Studies had moderate to high risk bias with incomplete descriptions of study methodology, populations, treatments, follow up, and outcomes. We conclude that observational studies reported similar improvements in HbA1c with CSII and MDI during pregnancy, but evidence was insufficient to rule out possible important differences between CSII and MDI for maternal and fetal outcomes. This highlights the need for future studies to examine the effectiveness and safety of CSII with insulin analogs and MDI in pregnant women with diabetes mellitus.
Introduction
In pregnant women with preexisting type 1 or type 2 diabetes, poor glycemic control is associated with poor pregnancy outcomes. Hyperglycemia at conception and in early pregnancy is associated with congenital anomalies.1 Hyperglycemia later in pregnancy is associated with fetal macrosomia, which can result in dysfunctional labor, cesarean delivery, birth injury, stillbirth, shoulder dystocia and neonatal hypoglycemia.2,3 In an effort to avoid these complications, physicians recommend tight glycemic control prior to conception and especially during pregnancy. A continuing challenge for perinatal providers is achieving tight glycemic control in patients with type 1 and type 2 diabetes without increasing the risk of maternal hypoglycemia.
To achieve tight glycemic control, physicians have traditionally used multiple daily injections of insulin (MDI) or continuous subcutaneous insulin infusion (CSII) with regular insulin. Rather than checking hemoglobin A1c (HbA1c) every 3 months, self-monitored fasting and 1-hour or 2-hour post-prandial glucose levels Are assessed daily by the patient and reviewed weekly by their physicians to ensure timely therapeutic changes to maintain tight control in pregnant women.4 The American Association of Clinical Endocrinologists recommends CSII for women with preexisting type 1 diabetes who are pregnant or considering pregnancy;5 however, it is unclear if these insulin delivery and glucose monitoring methods have any advantage over MDI and self-monitoring of blood glucose, respectively. One systematic review (n=5 trials; 154 pregnancies) comparing CSII with MDI in pregnant women with preexisting type 1 or type 2 diabetes found little evidence to support the use of one particular form of insulin delivery over another.6 While a prior systematic review found no substantial differences in short-term outcomes with CSII versus MDI, there were still gaps in knowledge about some fetal and maternal outcomes. An updated review was necessary to determine whether the addition of newly published studies would better inform the assessment of harms for both the mother and developing fetus. It was important to determine whether recent studies included long-term maternal outcomes or fetal outcomes such as growth in infants up to 1 year. Maternal outcomes of diabetes management in the reproductive years may have important implications for a woman's health later in her life course.
Because of the constant challenge of achieving glucose control in pregnancies complicated by preexisting diabetes, there is growing interest in the use of insulin analogues among pregnant women with type 1 or type 2 diabetes. In contrast to no-pregnant adults, the use of insulin analogs during pregnancy represents a sharp departure from the standard management of pregnancies complicated by type 1 diabetes. To date, however, the comparative benefits and harms of MDI and CSII with insulin analogues in pregnancy are largely unknown. Furthermore, the United States Food and Drug Administration approved insulin Levemir in pregnancy as a basal insulin analogue, supporting the importance of studying the effects of MDI and CSII with insulin analogues in pregnancy.7 It is important for perinatal clinicians to not only be aware of differences (e.g., glycemic control) in the effectiveness of CSII using insulin analogues and MDI, but to also understand the potential adverse effects for both mother and fetus.
Our objectives in this paper are to systematically review the differences in benefits and harms of MDI versus CSII with insulin analogues on short term and long term (up to 1 year) maternal and fetal outcomes in pregnant women with pre-existing diabetes.
Methods
Data sources and searches
We conducted this review as part of a larger project supported by the Agency for Healthcare Research and Quality (AHRQ); details of the methods can be found in the full report8,9 or in the protocol, which was published at effectivehealthcare.ahrq.gov. For the larger project, we initially searched for original studies in Medline®, Embase®, and the Cochrane Central Register of Controlled Trials in July 2011. Our search string included medical subject headings and text terms related to diabetes mellitus and insulin delivery, and was not limited by language. In May 2013, we searched the three databases again, this time using medical subject headings and text terms focused on finding studies of pregnant women with preexisting diabetes mellitus (see Appendix Table A1). We also searched the reference lists of included articles and relevant systematic reviews.
Data synthesis and analysis
Two authors independently reviewed citations for eligible studies. We included studies of pregnant women with preexisting diabetes mellitus. We included studies that compared CSII with MDI (defined as at least three injections per day). We included studies that used insulin analogues in the CSII arm, and long- and rapid-acting analogues and/or neutral protamine Hagedorn (NPH) and regular insulin or insulin analogue in the MDI arms because these insulin types are used in clinical practice. In our primary review we have excluded studies in which regular insulin was used in the CSII group because this is not the preferred clinical practice.10,11 To be consistent with that we excluded studies that used regular insulin in the CSII arm in this population.10,11 Analogue insulin is found to be safe and is recommended for pregnant women with diabetes mellitus.3,4 We searched for randomized controlled trials (RCTs) and observational studies with a concurrent comparison group that evaluated maternal and neonatal outcomes.
Maternal outcomes included intermediate outcomes (HbA1c, hyperglycemia, weight gain, and hypoglycemia frequency); severe hypoglycemia; cesarean delivery rates; quality of life; microvascular disease (retinopathy, nephropathy, and neuropathy); ketoacidosis; mortality; and process measures (ratio of basal to bolus insulin, frequency of adjusting insulin therapy, adherence to insulin therapy, and frequency of professional or allied health visits). Process measures are important, as they may impact how the insulin delivery and glucose monitoring methods affect the clinical outcomes.
Neonatal outcomes included gestational age, birth weight, neonatal hypoglycemia, major and minor anomalies, admission to a neonatal intensive care unit, stillbirth, neonatal mortality, and perinatal mortality. We excluded studies that were conducted in an inpatient setting or if the patients used the treatment device for less than 24 hours. Conflicts regarding article inclusion were resolved through consensus adjudication.
Using standardized data extractions forms, one reviewer extracted information on study characteristics (e.g., design, study period, follow up); study participants (e.g., age, gender, race, baseline HbA1c, weight, type of diabetes, and duration of diabetes); eligibility criteria; interventions [device model, type of insulin, MDI schedule, length of technology use, changes in insulin type used, patients/staff training, timing of treatment initiation in relation to pregnancy (prenatal, first trimester, second trimester), adherence to wearing the device]; definitions; and outcome measures, including measures of variability. A second reviewer checked abstracted data for completeness and accuracy.
Two reviewers independently assessed study quality. The quality assessment of observational studies was based on the Downs and Black quality checklist.12 We conducted separate meta-analyses when possible based on the type of insulin used in the MDI arm. It was done when there were at least two studies that were fairly comparable with respect to study design, population characteristics, and study duration. For continuous outcomes, we calculated a weighted mean difference in change scores between groups using a random-effects model, and for dichotomous outcomes, using a combined relative risk using the DerSimonian and Laird method.13 Heterogeneity among trials was tested using a chi-squared test (α≤0.10) or an I2 statistic (>50%).14 Meta-analyses were conducted using STATA (Intercooled, version 9.2, StataCorp, College Station, TX). We qualitatively summarized studies that were not amenable to pooling.
We graded the strength of the evidence by adapting a scheme recommended in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews.15 We graded the evidence for each treatment comparison for each outcome. We assessed the strength of evidence by assessing risk of bias, consistency, directness, precision, publication bias, and the magnitude of the effect.
Results
We retrieved 7,118 unique records from the original and updated searches.8 We reviewed 753 articles for inclusion (see Fig. 1). Of these, seven observational studies addressed the study questions among pregnant women, and all evaluated CSII versus MDI therapy in pregnant women with preexisting type 1 diabetes. One was a prospective study16 and six were retrospective cohort studies.17–22 We did not identify any studies conducted primarily among pregnant women with preexisting type 2 diabetes, and none of the studies in pregnant women were randomized clinical trials.
FIG. 1.
Summary of the literature search. *Total may exceed number in corresponding box, as articles could be excluded for more than one reason at this level. †Data from 35 studies in 38 publications: 28 compared MDI with CSII (9 in children and adolescents with type 1 diabetes; 9 in adults with type 1 diabetes; 4 [5 publications] in adults with type 2 diabetes); 9 (10 publications) compared rt-CGM with SMBG; 4 (5 publications) compared a sensor-augmented pump with MDI/SMBG. CENTRAL, Central Register of Controlled Trials; CSII, continuous subcutaneous insulin infusion; hrs, hours; MDI, multiple daily injections; rt-CGM, real-time continuous glucose monitor; SMBG, self-monitoring of blood glucose.
None of the studies evaluated maternal mortality, microvascular disease, quality of life, process measures, or birth trauma.
Qualitative summary
One study enrolled patients from an outpatient clinic,18 two studies enrolled from diabetes ob-gyn/pregnancy clinics,16,20 and another study enrolled patients from a university clinic (see Table 1).17 One study did not report where patients were enrolled.19 One study specifically stated that patients were managed by a team including endocrinologists and obstetricians/gynecologists.17 Studies were conducted in Italy,18,19 India,21 Poland,17,22 United Kingdom,16 and Spain.20 Women were given the choice to select either MDI or CSII in one study.16 Studies did not report relevant details of study design uniformly.
Table 1.
Study Population Characteristics of Studies Comparing Continuous Subcutaneous Insulin Infusion with Multiple Daily Injections Among Pregnant Women with Diabetes Mellitus
| Author, year | Design | Study duration | Study location | Study setting | Inclusion | Duration of diabetes (years) | Withdrawals (n) | Baseline HbA1c (%) | No. in each group | Age (years) | Type of insulin used | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruttomesso et al., 201119 | Cohort | NR | Italy | Four Italian centers | T1DM, adults, pregnant women only | MDI: mean, 13.5 CSII: mean, 16.5 |
NR | MDI: mean, 7.66 CSII: mean, 7.20 |
MDI: 44 CSII: 100 |
NR | MDI: prandial: aspart, lispro; basal: glargine CSII: prandial: lispro, aspart |
HbA1c (%), birth trauma (shoulder dystocia), NICU admission, gestational age (weeks), cesarean delivery, frequency of neonatal hypoglycemia, birth weight (g) |
| Chico et al., 201120 | Cohort | NR | Europe | Diabetes referral clinic, Ob/Gyn clinic | T1DM, adults, pregnant women only | MDI grp1: mean, 12 MDI grp 2: mean, 8.5 CSII: mean, 16 |
NR | MDI grp1: mean, 6.03 MDI grp 2: mean, 6.3 CSII: mean, 6.5 |
MDI grp 1: 196 MDI grp 2: 16 CSII: 59 |
MDI grp 1: range, 18–43 MDI grp2: range, 26–39 CSII: range, 25–42 |
MDI: prandial: regular insulin; basal: NPH CSII: prandial: lispro |
HbA1c (%), weight gain (kg), frequency of neonatal hypoglycemia, major anomalies, cesarean delivery |
| Cypryk et al., 200817 | Cohort | 36 weeks | Poland | Referral clinic | T1DM, adults, pregnant women only | MDI: mean, 7.7 CSII: mean, 12.7 |
NR | NR | MDI: 86 CSII: 30 |
NR | MDI: prandial: 30% used insulin lispro and 70% used regular insulin; basal: NPH CSII: prandial: 90% used insulin lispro; 10% not reported |
Birth weight, cesarean delivery, frequency of neonatal hypoglycemia, gestational age, HbA1c (%), minor anomalies, severe hypoglycemia |
| Kernaghan et al., 200816 | Cohort | 40 weeks | UK | Diabetic pregnancy clinic | T1DM & T2DM, adults, pregnant women only | NR | NR | MDI: mean, 8.01 CSII: mean, 7.62 |
MDI: 18 CSII: 24 |
NR | MDI: prandial: short-acting or rapid-acting insulin; basal: unspecified CSII: NR |
Birth weight, gestational age, HbA1c (%), minor anomalies |
| Volpe et al., 201018 | Cohort | 36.4 weeks | Italy | Outpatient clinic | T1DM, adults, pregnant women only | MDI: 12.1 CSII +SMBG: 16 |
NR | NR | MDI: 22 CSII+SMBG: 20 |
NR | MDI: prandial: short-acting insulin analogue; basal: NPH CSII: NR |
Birth weight, cesarean delivery, frequency of neonatal hypoglycemia, gestational age, HbA1c (%), major anomalies, minor anomalies, nephropathy, NICU admission, retinopathy, severe hypoglycemia, weight gain |
| Talaviya et al., 201321 | Cohort | 9 months | India | Referral clinic | T1DM adults, pregnant women only | MDI: 8.4 CSII: 8.5 |
NR | NR | MDI: 20 CSII: 14 |
MDI: 30.2 CSII: 31.3 |
MDI: rapid-acting insulin analogue CSII: lispro or aspart |
Birth weight, cesarean delivery, congenital anomalies, gestational age, HbA1c, neonatal hypoglycemia, preterm delivery |
| Wender-Ozegowska et al., 201322 | Cohort | 9 months | Poland | Ob/Gyn clinic | T1DM adults, pregnant women only | NR | NR | MDI: 7.1 CSII: 7.5 |
MDI: 64 CSII: 64 |
MDI: 27.2 CSII: 27.5 |
MDI: NPH CSII: lispro |
Birth weight, cesarean delivery, congenital anomalies, fetal mortality, gestational age, HbA1c, neonatal hypoglycemia, preterm delivery, weight gain |
CSII, continuous subcutaneous insulin infusion; grp, group; HbA1c, hemoglobin A1c; MDI, multiple daily injection; NA, not applicable; NICU, neonatal intensive care unit; NPH, Neutral Protamine Hagedorn; NR, not reported; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; UK, United Kingdom.
The number of participants per arm ranged from 14 to 86 pregnant women.16–22 Three studies reported having 100% Caucasian women.17,18,22 Women entered the studies at various stages of pregnancy. All study participants had preexisting type 1 diabetes, except that one study reported that one of the 17 pregnant women in the CSII arm had preexisting type 2 diabetes, and one of the 23 women in the MDI arm had type 2 diabetes.16 Two studies reported that CSII was started 6 months before participants became pregnant.18,19 One study reported enrolling participants 3 or 6 months before conception,21 and another reported enrolling them during first trimester.22 One study reported enrolling some of the study participants on CSII before pregnancy.17
The mean age of study populations ranged from 26 to 31.34 years. The mean HbA1c during the first trimester ranged from 6.9% to 7.8%,16–22 and the mean body mass index, reported in seven studies, ranged from 21.8 to 26.19 kg/m2. Baseline body mass index did not differ by arm.17–19 The duration of diabetes was reported in five studies and ranged from 7.7 to 16.5 years.17–19,21 One study reported that diabetes was diagnosed at the age of 14 years for both groups.22 Studies did not report on withdrawals. Insulin treatments varied across studies. Five studies reported that insulin lispro was used primarily for the CSII arm,16,17,19,20,22 and the type of insulin was not specified in the CSII arm in one study.18 We tried to contact the authors to confirm the type of insulin used in the CSII arm, but we did not receive a response. Since insulin analogs were used in the MDI arm, we assumed that insulin analogs were used in the CSII arm as well. One study reported using either Lispro or Aspart in the CSII arm.21
In the MDI groups, NPH insulin was used in four studies17,18,20,22 and insulin glargine was used in two other studies.16,19 One study reported using rapid acting insulin analog in the MDI arm.21 Two studies reported using four or more insulin injections daily in the MDI arms.16,18 One study had a total of four arms, MDI and CSII arms using regular insulin and MDI and CSII arms using insulin lispro. We included the lispro-based arms for our analyses to be consistent with other included studies.20 Four studies reported on the provision of training prior to initiating insulin pump therapy in the CSII arms.16,18,20,22 The mean duration of therapy was reported in three studies and ranged from 36 to 40 weeks.16–18
Reported glycemic targets varied across studies. One study specified a HbA1c target of 6.5%,16 one a preprandial blood glucose target of 4.99 mmol/L (90 mg/dL) and a postprandial blood glucose target of 7.2 mmol/L (130 mg/dL),18 and another a preprandial blood glucose target of 3.3 to 4.99 mmol/L (59.4 to 90 mg/dL).17 One study reported a fasting blood sugar target of 5 mmol/L (90.1 mg/dL) and 2 hours postprandial target of 6.7 mmol/L (120.7 mg/dL).22 Only one study reported on guidelines for management of blood glucose between visits.16
Maternal outcomes
HbA1c
All seven studies reported an improvement in HbA1c in both the CSII and MDI groups during pregnancy without any significant difference between groups in HbA1c or substantial differences across the three trimesters of pregnancy (see Table 2). The mean between-group differences in third trimester HBA1C values in each of the studies were 0.2 (95% confidence interval [95% CI], −0.3 to 0.7),18 0.6 (95% CI, −0.7 to 1.9),16 −0.3 (95% CI, −0.6 to −0.03),19 −0.4 (95% CI, −0.8 to 0.04),17 and 0.4 (95% CI, −0.9 to 1.7).20 One study reported a significant (p<0.05) reduction In HBA1C level compared with baseline in each trimester in both groups. There was statistically insignificant but higher reduction in HBA1C in the CSII treated group in all three trimesters compared with the MDI treated group.21 Another study reported a significant reduction in HBA1C level during pregnancy in the CSII group compared with the MDI group, particularly in women with the highest HBA1C concentrations in the first trimester.22 We did not perform a meta-analysis, because only two studies reported baseline mean HbA1c. One was a retrospective study and the other was a prospective study.16,19
Table 2.
Differences in HbA1c by Trimester in the CSII and MDI Arms in Women with Preexisting Type 1 Diabetes
| Author, year | Intervention arms (n) | HbA1c (%) first trimester | HbA1c (%) second trimester | HbA1c (%) third trimester | Statistical difference between groups |
|---|---|---|---|---|---|
| Volpe et al., 201018 | MDI, 22 | 7.4 | – | 6.1 | NS |
| CSII, 20 | 6.9 | – | 6.3 | ||
| Cypryk et al., 200817 | MDI, 86 | 7.8 | 6.7 | 6.8 | NS |
| CSII, 30 | 7.4 | 6.5 | 6.4 | ||
| Kernaghan et al., 200816 | MDI, 18 | 7.3 | 6.6 | 6.44 | NS |
| CSII, 24 | 6.95 | 6.3 | 6.63 | ||
| Bruttomesso et al., 201119 | MDI, 44 | 7.2 | 6.7 | 6.5 | NS |
| CSII, 100 | 6.6 | 6.1 | 6.2 | ||
| Chico et al., 201120 | MDI, 16 | 6.1 | 5.8 | 5.9 | NS |
| CSII, 59 | 6.3 | 6.0 | 6.3 | ||
| Talaviya et al., 201321 | MDI, 20 | 7.8 | 7.5 | 7.2 | NS |
| CSII, 14 | 7.8 | 7.2 | 6.7 | ||
| Wender-Ozegowska et al., 201322 | MDI, 64 | 7.1 | 6.2 | 6.3 | NS |
| CSII, 64 | 7.5 | 6.6 | 6.3 |
Cesarean delivery
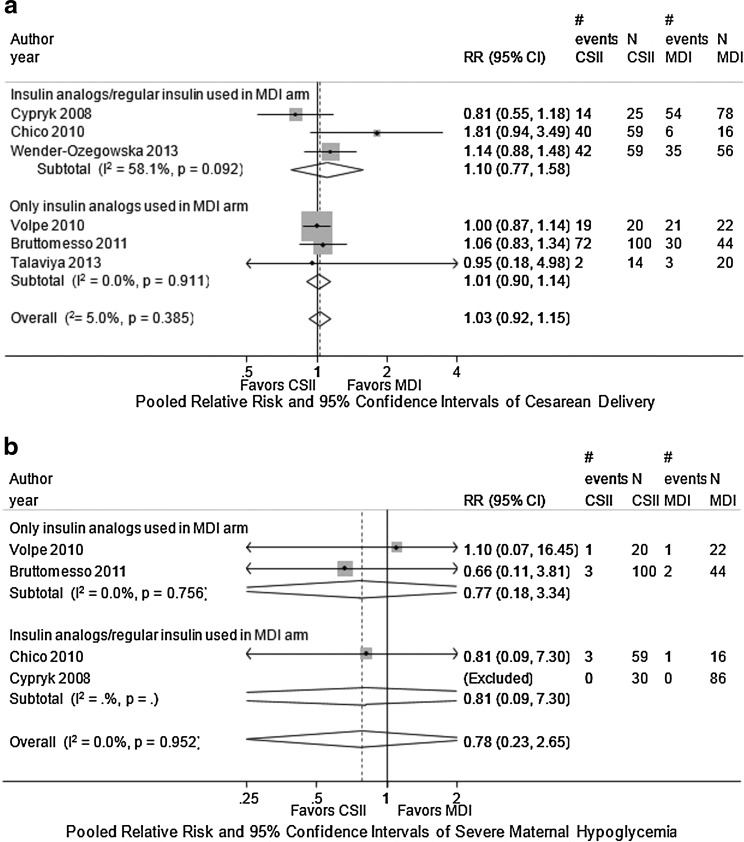
Six studies reported on the rate of cesarean delivery but did not distinguish between elective, repeat, or other causes for cesarean delivery (see Table 3).17–22 One study found a higher rate of cesarean delivery in women in the CSII arm compared with women in the MDI arm, but the study did not provide data on further analysis.20 One study reported a very high rate of cesarean delivery in both comparison groups: 71% in CSII group and 62% in MDI group.22 Meta-analysis of the three retrospective studies comparing CSII with MDI found that used insulin analogues in the MDI arm showed a combined relative risk of 1.01 (95% CI, 0.9 0 to 1.14; see Fig. 2a).18,19,21 We found no evidence of statistical heterogeneity (I2 of 0%). Meta-analysis of three studies that allowed regular insulin to be used in the MDI arm did not change the results, although statistical heterogeneity was high (I2=58.1%).
Table 3.
Rates of Cesarean Section Between MDI and CSII Arms in Women with Preexisting Type 1 Diabetes
| Author, year | MDI rate of cesarean section | CSII rate of cesarean section | Statistical significance |
|---|---|---|---|
| Volpe et al., 201018 | 94% | 95% | NS |
| Cypryk et al., 200817 | 46% | 69.2% | 0.235 |
| Bruttomesso et al., 201119 | 73.2% | 77.4% | NS |
| Chico et al., 201120 | 38.5% | 67.6% | – |
| Talaviya et al., 201321 | 15% | 14% | 0.82 |
| Wender-Ozegowska et al., 201322 | 63% | 71% | NS |
FIG. 2.
Combined relative risk of maternal outcomes in MDI versus CSII interventions among pregnant women with preexisting type 1 diabetes. (a) Combined relative risk of cesarean delivery in MDI versus CSII interventions among pregnant women with preexisting type 1 diabetes. (b) Combined relative risk of severe maternal hypoglycemia in MDI versus CSII interventions among pregnant women with preexisting type 1 diabetes. 95% CI, 95% confidence interval; RR, relative risk.
Maternal hypoglycemia
Four studies compared the number of severe hypoglycemic events in women using CSII versus MDI.17–20 Another study reported the following about severe hypoglycemia: “Indeed, the number of severe hypoglycemic episodes decreased significantly in CSII patients during pregnancy, although minimal glucose values were similar in both groups.” The study did not report actual numbers, so this study was not included in the meta-analysis.22 A meta-analysis of two retrospective cohort studies that used insulin analog in the MDI arm showed no difference in the rate of maternal hypoglycemia for CSII compared with MDI: combined relative risk of 0.77 (95% CI, 0.18 to 3.34; see Fig. 2b).18,19 We found no evidence of statistical heterogeneity, and no single study influenced the results. Including studies that allowed regular insulin to be used in the MDI arm did not change the results.
Maternal weight gain
Three studies measured weight gain in pregnant women with preexisting diabetes treated with MDI and CSII.18,20,22 The difference in weight gain between the CSII and MDI treatment arms was not statistically significant in these studies.18,20
Ketoacidosis
One study reported diabetic ketoacidosis in pregnant women with preexisting diabetes treated with MDI and CSII.19 This study reported that there were two episodes (4.7%) in the MDI arm and one episode (1.1%) in the CSII arm.19
Neonatal outcomes
Gestational age at delivery
Six studies reported on gestational age at delivery and found no significant difference between the MDI and CSII groups. Gestational age at delivery ranged from 36.3 weeks to 38 weeks in the MDI arms and 36.3 weeks to 38 weeks in the CSII arms.16–19,21,22
Neonatal hypoglycemia
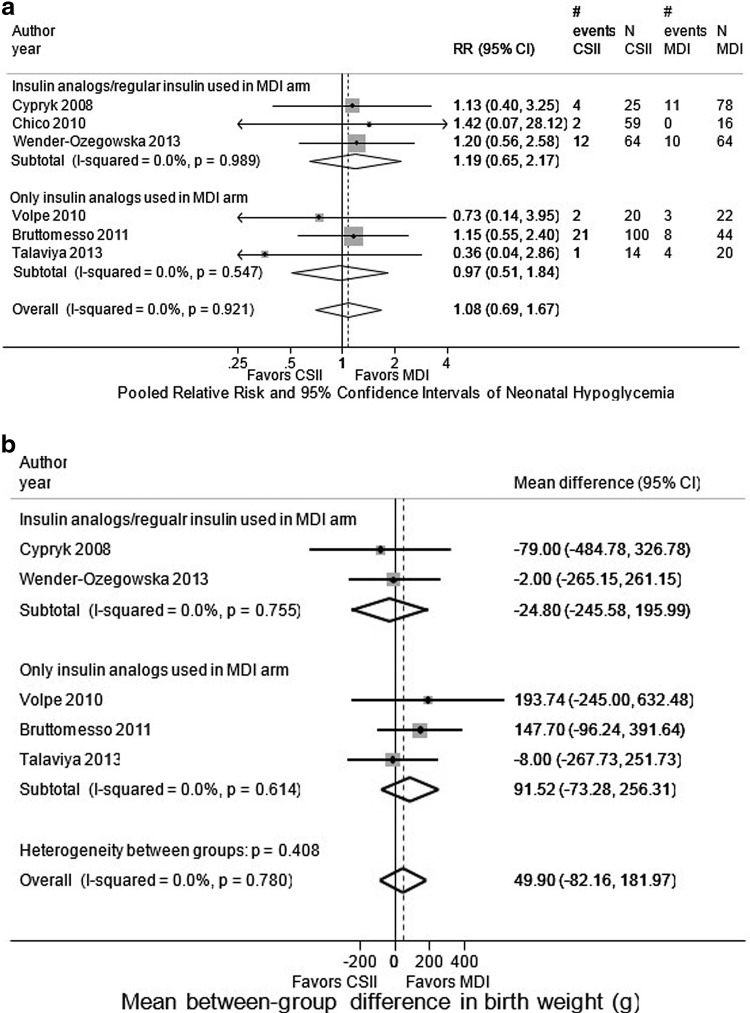
Six studies reported rates of neonatal hypoglycemia.17–22 This was defined as a blood glucose less than 2.2 mmol/L (or less than 40 mg/dL) in four studies17,19,20,22 and was not defined in two studies.18,21 Meta-analysis of three retrospective cohort studies that used only insulin analogues in the MDI arm for frequency of neonatal hypoglycemia showed a combined relative risk of neonatal hypoglycemia for CSII compared with MDI of 0.97 (95% CI, 0.51 to 1.84; see Fig 3a).18,19,21 We found no evidence of statistical heterogeneity, and no single study significantly influenced results. Meta-analysis of three retrospective cohort studies that allowed regular insulin in the MDI arm showed a combined relative risk for neonatal hypoglycemia for CSII compared with MDI of 1.19 (95% CI, 0.65 to 2.17; see Fig. 3a).17,20,22
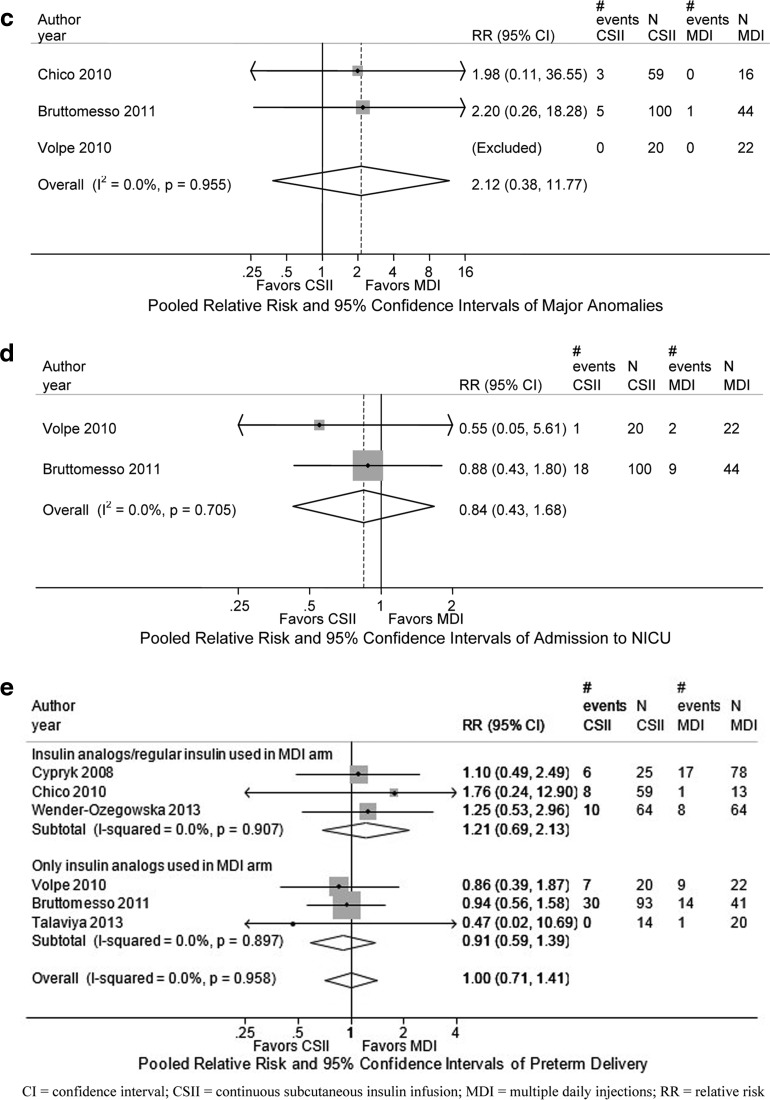
FIG. 3.
Combined relative risk of neonatal outcomes in MDI versus CSII interventions among pregnant women with preexisting type 1 diabetes. (a) Combined relative risk of neonatal hypoglycemia comparing CSII with MDI among pregnant women with preexisting diabetes. (b) Combined mean between-group difference between MDI and CSII in birth weight among infants born to women with preexisting type 1 diabetes. (c) Combined relative risk of major congenital anomalies in MDI versus CSII interventions among infants born to women with preexisting type 1 diabetes. (d). Combined relative risk of neonatal intensive care unit admission in MDI versus CSII interventions among infants born to women with preexisting type 1 diabetes. (e) Combined relative risk of preterm delivery in MDI versus CSII interventions among pregnant women with preexisting type 1 diabetes.
Birth weight
Five studies reported on mean birth weight, which ranged from 3101 to 3767 grams (see Table 4).17–19,21,22 Meta-analysis of three retrospective cohort studies that used only insulin analog in the MDI arm showed a combined mean between-group difference in birth weight for CSII compared with MDI of 91.52 g, but this difference was not statistically significant (95% CI, −73.28 to 256.31 g; see Fig. 3b).18,19,21 Meta-analysis of two retrospective cohort studies that allowed regular insulin in the MDI arm showed a combined mean between-group difference in birth weight for CSII compared with MDI of −24.80 g, but this difference was not statistically significant (95% CI, −245.58 to 195.99 g; see Fig. 3b).17,22 We found no evidence of statistical heterogeneity, and no single study significantly influenced the results.
Table 4.
Neonatal Birth Weights in the CSII and MDI Arms in Women with Preexisting Type 1 Diabetes
Congenital anomalies
Three studies reported on major congenital anomalies in pregnant women treated with CSII versus MDI.18–20 Women initiated CSII therapy prior to pregnancy in these studies.18–20 Major congenital anomalies were defined as life-limiting, requiring surgery, or causing significant functional or cosmetic impairment,20 or following the European Registration of Congenital Anomalies and Twins (EUROCAT) classification,19 or not further specified.18 Two studies reported on congenital anomalies. One shows no evidence of any congenital anomaly in either group,21 and the other reported four (6.2%) in CSII group and two (3.1%) in MDII group.22 Both studies didn't have a definition for congenital anomalies.
We performed meta-analysis for two retrospective cohort studies and it showed a combined relative risk of 2.12 favoring MDI that was not significant (95% CI, 0.38 to 11.77, Fig. 3c).19,20 We did not find evidence of statistical heterogeneity.
Three studies reported on minor congenital anomalies. None of the studies defined minor congenital anomalies. There were no minor congenital anomalies in either group in two studies,16,18 and the rate of minor congenital anomalies were 2.3% (2/86 patients) in the MDI group and 13% (4/30 patients) in the CSII group in the other study (p=0.05).17
Neonatal intensive care unit admission
Two studies reported on neonatal intensive care unit admissions rates.18,19 These ranged from 9% to 35% in the MDI groups and 5% to 33% in the CSII groups.18,19 Meta-analysis of these two retrospective cohort studies comparing CSII with MDI showed a combined relative risk of 0.84 (95% CI, 0.43 to 1.68; Fig. 3d).18,19 There was no evidence of statistical heterogeneity, and no evidence of publication bias.
Preterm delivery
In the six studies reporting on preterm delivery, the definition was not uniform. Preterm delivery rates ranged from 7.7% to 40% in the MDI groups and 0% to 33% in the CSII groups (see Table 5).17–22 Meta-analysis of the three retrospective studies comparing CSII with MDI with insulin analogues showed a combined relative risk of 0.91 (95% CI, 0.59 to 1.39; see Fig. 3e).18,19,21 Meta-analysis of the three retrospective studies comparing CSII with MDI with regular insulin showed a combined relative risk of 1.21 (95% CI, 0.69 to 2.13; see Fig. 3e).17,20,22
Table 5.
Rates of Preterm Delivery Between MDI and CSII Arms in Women with Pre-existing Type 1 Diabetes
Stillbirth rates and neonatal and perinatal mortality
Five studies reported on stillbirth rates. Three reported no stillbirths in either group,16,18,20 and one study reported having one stillbirth in the MDI group.17 Another study reported having two intrauterine deaths (after the 22 week) in each group.22 Three studies reported on neonatal mortality rate. One neonatal death occurred in both arms of one study,17 no neonatal deaths in either group in another,16 and a 0% neonatal mortality rate in the MDI group and 2.7% rate in the CSII group in a third study.20 One study reported on perinatal mortality rate. This study reported a 0% perinatal mortality rate in the MDI group and 2.7% rate in the CSII group.20
Summary
The strength of evidence examining the comparative effectiveness of CSII versus MDI in women with preexisting type 1 diabetes was low for the outcome of HbA1c and insufficient for the other outcomes, limiting our ability to assess differences in outcomes for the two insulin delivery modalities (see Appendix Table A2). The strength of evidence was considered low because the evidence was limited to observational studies. There were no RCTs that met our eligibility criteria. Also, for the outcomes examined, data were insufficient to determine the precision of effect estimates.
Discussion
Main findings
Our systematic review of observational studies showed no difference in HbA1c lowering between CSII and MDI treatment in pregnant women with type 1 diabetes. However, the strength of this evidence was low due to the risk of bias and lack of precision in outcome estimates. The evidence was insufficient to draw definitive conclusions about other maternal and fetal outcomes for pregnant women with type 1 diabetes. Results were similar when CSII was compared with MDI with insulin analogs or regular insulin.
A prior systematic review and meta-analysis of six randomized clinical trials comparing CSII using regular insulin and MDI in pregnant diabetic women also showed that pregnancy outcomes and glycemic control were not significantly different among these two groups. While the CSII group had a higher number of ketoacidosis episodes and diabetic retinopathy, the difference was not statistically significant. These findings were similar to ours despite differences in inclusion criteria (e.g., CSII using regular insulin in the prior study). The authors concluded that it is necessary to conduct large multicenter trials to examine maternal and fetal outcomes.23 Effective glucose control in pregnancies complicated by type 1 and type 2 diabetes has important short-term and lifetime clinical implications for the expectant mother and developing fetus. Treatment outcomes during pregnancy are thought to offer a view of potential complications across the life course for mother and child.24 This life course model provides a framework in which to view the comparative effectiveness and safety of newer methods for insulin delivery and glucose control (i.e., CSII) compared with traditional insulin delivery methods. It is well established that tight glucose control during pregnancy reduces the short-term risk of adverse delivery outcomes, such as macrosomia, fetal growth restriction and cesarean delivery.
There is also an emerging chain of data indicating that the intrauterine hormonal milieu in diabetic pregnancies can influence fetal development and programming, altering fetal metabolism and increasing the risk of chronic disease in the offspring.25 Efforts that compare the effectiveness of insulin delivery in pregnancies complicated by type 1 and especially type 2 diabetes provide important data that can be directly translated into clinical care. Also, such data can inform guidelines for future perinatal care services and improve the current paradigm of perinatal practice.
Strengths and limitations
Most of the observational studies included in this review had important limitations.16–21 All studies provided limited descriptions of study setting, study population, treatment, follow up and outcomes. One study did not report type of insulin used in the CSII arm (although in that study insulin analog had been used in the MDI arm), and none of the studies described details of losses to follow-up. Most studies did not report the racial and ethnic composition of the study populations. For those that did provide this information, the majority of participants were Caucasian, likely reflecting the fact that type 1 diabetes is much rarer in minority populations. Since no studies focused on pregnant women with preexisting type 2 diabetes, we were unable to draw conclusions about the effectiveness of insulin delivery methods in this population. Studies used heterogeneous definitions of hypoglycemia, hyperglycemia, and weight gain, preventing us from combining data to determine effect estimates for some of these intermediate outcomes. None of the studies included data on long-term macrovascular complications of diabetes. This is likely related to the fact that these complications develop over many years, and studies generally only followed subjects during pregnancy.
Several limitations of our systematic review deserve mention. First, systematic reviews are limited by the state of the available literature. Notably, our evidence synthesis is based on observational studies and not RCTs. Thus, our findings are particularly susceptible to confounding and selection bias. Second, the applicability or generalizability of any systematic review is constrained by the criteria used to select studies for inclusion. Third, unlike previous reviews on this topic we have excluded studies that used regular insulin in the CSII arm from our meta-analysis, leaving us with only a limited number of studies with small sample size for each outcome. Finally, while our search strategy was comprehensive and included non-English language publications, we may have missed studies that have not yet been reported in a peer-reviewed journal. Finally, our study did not address the availability, costs and insurance coverage of CSII, which may be obstacles to their use. In general, insulin pumps cost between $6,000 and $7,000, with supply costs of approximately $2,000 per year (Medtronic, Animas, and OmniPod Personal communications). The extent to which insurance covers these costs will contribute to their use in practice.
Interpretation
From this systematic review of observation studies we found that CSII and MDI do not affect HbA1c differentially in pregnant women with preexisting type 1 diabetes with low strength of evidence.
Conclusions
Our report highlights that the systematic review of available observational studies may not facilitate making clinical decision to choose CSII or MDI for pregnant women with preexisting type 1 diabetes. This report also shows the need for several areas of future research examining the effect of insulin delivery in the management of preexisting diabetes mellitus in pregnant women. We identified a need for well-conducted RCTs of intensive insulin therapy delivered via CSII versus MDI in pregnant women with both type 1 and type 2 diabetes. In addition to HbA1c, other important outcomes to examine include maternal outcomes (cesarean delivery, hypoglycemia, weight gain, quality of life, and mortality) and neonatal outcomes (gestational age at delivery, hypoglycemia, birth weight, congenital anomalies, neonatal intensive care unit admission, preterm delivery, stillbirths, neonatal and perinatal mortality, and birth trauma). Also, to allow cross-comparisons, future RCTs and observational studies should use a uniform definition of hypoglycemia, preferably that recommended by the American Diabetes Association.26 Finally, cost-effectiveness of CSII versus MDI should be examined in future research studies.
Appendix
Table A1.
PubMed Search Strategy
| Search | Terms |
|---|---|
| Original search conducted in July 2011 | ((“Diabetes Mellitus”[mh] OR Diabet*[tiab] OR hyperglycem*[tiab] OR hyperglycaem*[tiab]) AND (“Insulin Infusion Systems”[mh] OR “continuous subcutaneous insulin”[tiab] OR CSII[tiab] OR “insulin pump”[tiab] OR “insulin pumps”[tiab] OR “pump therapy”[tiab] OR “pump treatment”[tiab] OR “artificial pancreas”[tiab] OR (“Monitoring, Ambulatory”[mh] AND (glucose[tiab] OR insulin[tiab] OR glycem*[tiab] OR glycaem*[tiab])) OR “CGM”[tiab] OR (“continuous glucose”[tiab] AND (monitor*[tiab] OR sensing[tiab] OR sensor*[tiab])))) NOT (animal[mh] NOT human [mh]) |
| Updated search, focusing on pregnant women with preexisting diabetes mellitus | ((“Diabetes Mellitus”[mh] OR Diabet*[tiab] OR hyperglycem*[tiab] OR hyperglycaem*[tiab]) AND (“Insulin Infusion Systems”[mh] OR “continuous subcutaneous insulin”[tiab] OR CSII[tiab] OR “insulin pump”[tiab] OR “insulin pumps”[tiab] OR “pump therapy”[tiab] OR “pump treatment”[tiab] OR “artificial pancreas”[tiab] OR (“Monitoring, Ambulatory”[mh] AND (glucose[tiab] OR insulin[tiab] OR glycem*[tiab] OR glycaem*[tiab])) OR “CGM”[tiab] OR (“continuous glucose”[tiab] AND (monitor*[tiab] OR sensing[tiab] OR sensor*[tiab]))) AND (pregnancy[mh] OR pregnan*[tiab])) NOT (animal[mh] NOT human [mh]) |
Table A2.
Limitations on the Evidence of the Comparative Effectiveness of MDI Versus CSII in Pregnant Women with Preexisting Type 1 Diabetes
| Outcome | Precision | Consistency | No. of studies/No. of good quality studies | Main findings |
|---|---|---|---|---|
| Cesarean section rates | Precise | Consistent | 6 (All OBS)/0 | Meta-analysis of three retrospective cohort studies comparing CSII with MDI that used insulin analogues in the MDI arm showed a combined relative risk of 1.01 (95% CI, 0.90 to1.14; see Fig. 2a).18,19,21 Including studies that allowed regular insulin to be used in the MDI arm did not change the results. |
| Maternal hypoglycemia | Imprecise | Consistent | 4 (All OBS)/0 | Meta-analysis of two retrospective cohort studies showed no difference in the rate of maternal hypoglycemia for CSII compared with MDI: combined relative risk of 0.77 (95% CI, 0.18 to 3.34).18,19 Including studies that allowed regular insulin to be used in the MDI arm did not change the results. |
| Maternal weight gain | Cannot determine | Consistent | 3 (All OBS)/0 | There was no difference in weight gain between the CSII and MDI intervention groups in all three reported studies. The mean between-group difference in weight gain was 1.9 kg (95% CI, −0.9 to 4.7 kg) in one study.18 The other study reported a median weight gain of 13.5 kg in the CSII group and 13.9 kg in the MDI group.20 |
| Ketoacidosis | Imprecise | Unknown | 1 (All OBS)/0 | One study reported that there were two episodes of ketoacidosis (4.7%) in the MDI arm and one episode (1.1%) in the CSII arm.19 |
| Other maternal outcomes | NA | NA | 0/0 | We did not include any studies that evaluated maternal mortality, microvascular or macrovascular disease, QOL, or any of the process measures. |
| Gestational age at delivery | Cannot determine | Consistent | 5 (All OBS)/0 | Gestational age at delivery ranged from 36.3 weeks to 38 weeks for MDI and from 36.3 weeks to 38 weeks for CSII, and there was no significant difference between the MDI and CSII groups.16–19,21,22 |
| Neonatal hypoglycemia | Imprecise | Consistent | 6 (All OBS)/0 | Meta-analysis of three retrospective cohort studies that used only insulin analogues in the MDI arm for frequency of neonatal hypoglycemia showed a combined relative risk of neonatal hypoglycemia for CSII compared with MDI of 0.97 (95% CI, 0.51 to 1.84; see Fig. 3a).18,19,21 Meta-analysis of three retrospective cohort studies that allowed regular insulin in the MDI arm showed a combined relative risk for neonatal hypoglycemia for CSII compared with MDI of 1.19 (95% CI, 0.65 to 2.17; see Fig. 3a).17,20,22 |
| Birth weight | Cannot determine | Unknown | 5 (All OBS)/0 | Meta-analysis of three retrospective cohort studies that used only insulin analog in the MDI arm showed a combined mean between-group difference in birth weight for CSII compared with MDI of 91.52 g, but this difference was not statistically significant (95% CI, −73.28 to 256.31 g; see Fig. 3b).18,19,21 Meta-analysis of two retrospective cohort studies that allowed regular insulin in the MDI arm showed a combined mean between-group difference in birth weight for CSII compared with MDI of −24.80g, but this difference was not statistically significant (95% CI, −245.58 to 195.99 g; see Fig. 3b). |
| Major congenital anomalies | Imprecise | Unknown | 2 (All OBS)/0 | Meta-analysis for only two retrospective cohort studies for major congenital anomalies showed a pooled RR of 2.12 favoring MDI that was not significant (95% CI, 0.38 to 11.77).19,20 |
| Minor congenital anomalies | Cannot determine | Unknown | 3 (All OBS)/0 | Three studies found no difference in minor congenital anomalies between the MDI and CSII groups. There were no minor congenital anomalies in either group in two studies,16,18 and rates of minor congenital anomalies and pregnancy termination rates were 2.3% (2/86 patients) in the MDI group and 13% (4/30 patients) in the CSII group (p=0.05).17 |
| NICU admissions | Imprecise | Unknown | 2 (All OBS)/0 | Meta-analysis on two retrospective cohort studies for admission to the neonatal intensive care unit showed a pooled RR of 0.84 that was not significant (95% CI, 0.43 to 1.68).18,19 |
| Preterm delivery | Imprecise | Unknown | 6 (All OBS)/0 | Meta-analysis of the three retrospective studies comparing CSII with MDI with insulin analogues showed a combined relative risk of 0.91 (95% CI, 0.59 to 1.39; see Fig. 3e).18,19,21 Meta-analysis of the three retrospective studies comparing CSII with MDI with regular insulin showed a combined relative risk of 1.21 (95% CI, 0.69 to 2.13; see Fig. 3e).17,20,22 |
| Still birth rates | Cannot determine | Unknown | 4 (All OBS)/0 | Four studies reported on still birth rates. Three reported that there were no still births in either group,16,18,20 and one study reported having one still birth in MDI group.17 |
| Neonatal mortality | Cannot determine | Unknown | 3 (All OBS)/0 | Three studies reported on neonatal mortality rate. Each group had one neonatal death in one study,17 no neonatal deaths in either group in another,16 and a 0% neonatal mortality rate in the MDI group and 2.7% rate in the CSII group in a third study.20 |
| Perinatal mortality | Cannot determine | Unknown | 1 (All OBS)/0 | One study reported a 0% perinatal mortality rate in MDI group and a 2.7% rate in CSII group.20 |
| Birth trauma | NA | NA | 0 | We did not include any studies that reported on birth trauma. |
CI, confidence interval; CSII, continuous subcutaneous insulin infusions; g, grams; HbA1c, hemoglobin A1c; kg, kilograms; MDI, multiple daily injections; NICU, neonatal intensive care unit; OBS, observational study; QOL, quality of life; RR, relative risk.
The strength of the evidence was defined as follows: High, high confidence that the evidence reflects the true effect. Further research is unlikely to change our confidence in the estimate of the effect. Moderate, moderate confidence that the evidence reflects the true effect. Further research may change our confidence in the estimate of the effect and may change the estimate. Low, low confidence that the evidence reflects the true effect. Further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate. Insufficient, evidence is unavailable.
Acknowledgments
The authors would like to thank Dr. Christine Chang, Task Order Officer, for her support and guidance throughout the project.
PR, NM, WKN, HCY, TB, YS, LMW, EBN, ZB, EBB, and SHG made substantial contributions to the conception and design of the review. PR, NM, WKN, HCY, TB, YS, LMW, EBN, ZB, EBB, and SHG participated in the acquisition of the data. PR, NM, WKN, LMW, and SHG contributed to the analysis and interpretation of the data. PR, NM, WKN, and SHG drafted the article. PR, NM, WKN, HCY, TB, YS, LMW, EBN, ZB, EBB, and SHG reviewed and approved the manuscript.
Dr. Nicholson is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (DK095189-02). Grant support has been provided by AHRQ. The authors did not need to seek ethics approval for this study.
The authors of this article are responsible for its contents, including any clinical or treatment recommendations. No statement in this article should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
Author Disclosure Statement
None of the authors or anyone listed in the acknowledgements have a conflict of interest to disclose.
References
- 1.Kitzmiller JL, Gavin LA, Gin GD, Jovanovic-Peterson L, Main EK, Zigrang WD. Preconception care of diabetes. Glycemic control prevents congenital anomalies. JAMA 1991;265:731–736 [PubMed] [Google Scholar]
- 2.Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care 2008;31:1060–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Collaborating Centre for Women's and Children's Health (UK)Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. National Institute for Health and Clinical Excellence clinical guideline 63. London: RCOG Press, 2008. Available at: www.nice.org.uk/nicemedia/pdf/CG063Guidance.pdf Accessed November1, 2014 [PubMed] [Google Scholar]
- 4.American Congress of Obstetricians and Gynecologists (ACOG) Committee on Practice Bulletins. Clinical management guidelines for obstetrician-gynecologists. Number 60, March 2005. Pregestational diabetes mellitus. Obstet Gynecol 2005;105:675–685 [DOI] [PubMed] [Google Scholar]
- 5.Grunberger G, Bailey TS, Cohen AJ, et al. Statement by the American Association of Clinical Endocrinologists Consensus Panel on insulin pump management. Endocr Pract 2010;16:746–762 [DOI] [PubMed] [Google Scholar]
- 6.Farrar D, Tuffnell DJ, West J. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev 2007:CD005542. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration (FDA). Highlights of prescribing information: Levemire (insulin detemir [rDNA origin] injection) solution for subcutaneous injection. Silver Spring, MD: Health and Human Services, FDA, 2012. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2012/021536S039lbl.pdf Accessed November1, 2014 [Google Scholar]
- 8.Golden SH, Brown T, Yeh HC, et al. Methods for insulin delivery and glucose monitoring. Comparative effectiveness reviews, No. 57. Rockville MD: Agency for Healthcare Research and Quality (US), 2012 [PubMed] [Google Scholar]
- 9.Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: A systematic review and meta-analysis. Ann Intern Med 2012;157:336–347 [DOI] [PubMed] [Google Scholar]
- 10.DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. JAMA 2003;289:2254–2264 [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care 2012;35:S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: Grading the strength of a body of evidence when comparing medical interventions–agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol 2010;63:513–523 [DOI] [PubMed] [Google Scholar]
- 16.Kernaghan D, Farrell T, Hammond P, Owen P. Fetal growth in women managed with insulin pump therapy compared to conventional insulin. Eur J Obstet Gynecol Reprod Biol 2008;137:47–49 [DOI] [PubMed] [Google Scholar]
- 17.Cypryk K, Kosinski M, Kaminska P, Kozdraj T, Lewinski A. Diabetes control and pregnancy outcomes in women with type 1 diabetes treated during pregnancy with continuous subcutaneous insulin infusion or multiple daily insulin injections. Pol Arch Med Wewn 2008;118:339–344 [PubMed] [Google Scholar]
- 18.Volpe L, Pancani F, Aragona M, et al. Continuous subcutaneous insulin infusion and multiple dose insulin injections in Type 1 diabetic pregnant women: a case-control study. Gynecol Endocrinol 2010;26:193–196 [DOI] [PubMed] [Google Scholar]
- 19.Bruttomesso D, Bonomo M, Costa S, et al. Type 1 diabetes control and pregnancy outcomes in women treated with continuous subcutaneous insulin infusion (CSII) or with insulin glargine and multiple daily injections of rapid-acting insulin analogues (glargine-MDI). Diabetes Metab 2011;37:426–431 [DOI] [PubMed] [Google Scholar]
- 20.Chico A, Saigi I, Garcia-Patterson A, et al. Glycemic control and perinatal outcomes of pregnancies complicated by type 1 diabetes: Influence of continuous subcutaneous insulin infusion and lispro insulin. Diabetes Technol Ther 2010;12:937–945 [DOI] [PubMed] [Google Scholar]
- 21.Talaviya PA, Saboo BD, Joshi SR, et al. Pregnancy outcome and glycemic control in women with type 1 diabetes: A retrospective comparison between CSII and MDI treatment. Diabetes Metab Syndr 2013;7:68–71 [DOI] [PubMed] [Google Scholar]
- 22.Wender-Ozegowska E, Zawiejska A, Ozegowska K, et al. Multiple daily injections of insulin versus continuous subcutaneous insulin infusion for pregnant women with type 1 diabetes. Aust N Z J Obstet Gynaecol 2013;53:130–135 [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs intensive conventional insulin therapy in pregnant diabetic women: a systematic review and metaanalysis of randomized, controlled trials. Am J Obstet Gynecol 2007;197:447–456 [DOI] [PubMed] [Google Scholar]
- 24.Ali S, Dornhorst A. Diabetes in pregnancy: Health risks and management. Postgrad Med J 2011;87:417–427 [DOI] [PubMed] [Google Scholar]
- 25.Bush NC, Chandler-Laney PC, Rouse DJ, Granger WM, Oster RA, Gower BA. Higher maternal gestational glucose concentration is associated with lower offspring insulin sensitivity and altered beta-cell function. J Clin Endocrinol Metab 2011;96:E803–E809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]