Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is being applied more widely for oligometastatic disease. This technique is now being used for non-spine bony metastases in addition to liver, spine, and lung. However, there are few studies examining the toxicity and outcomes of SBRT for non-spine bone metastases.
Methods and Materials
Between 2008 and 2012, 74 subjects with oligometastatic non-spine bony metastases of varying histologies were treated at the Mayo Clinic with SBRT. A total of 85 non-spine bony sites were treated. Median local control, overall survival, and progression-free survival were described. Acute toxicity (defined as toxicity <90 days) and late toxicity (defined as toxicity ≥90 days) were reported and graded as per standardized Common Toxicity Criteria for Adverse Events 4.0 criteria.
Results
The median age of patients treated was 60 years. The most common histology was prostate cancer (31%) and most patients had fewer than 3 sites of disease at the time of simulation (64%). Most of the non-spine bony sites lay within the pelvis (65%). Dose and fractionation varied but the most common prescription was 24 Gy/1 fraction. Local recurrence occurred in 7 patients with a median time to failure of 2.8 months. Local control was 91.8% at 1 year. With a median follow-up of 7.6 months, median SBRT specific overall survival and progression-free survival were 9.3 months and 9.7 months, respectively. Eighteen patients developed acute toxicity (mostly grade 1 and 2 fatigue and acute pain flare); 9 patients developed grade 1–2 late toxicities. Two patients developed pathologic fractures but both were asymptomatic. There were no late grade 3 or 4 toxicities.
Conclusions
Stereotactic body radiation therapy is a feasible and tolerable treatment for non-spine bony metastases. Longer follow-up will be needed to accurately determine late effects.
Introduction
An important component of a clinical radiation oncology practice is the treatment of painful bony metastases. Multiple randomized trials have shown that external beam radiation with a single 8 Gy fraction is effective for pain control although the need for retreatment is more frequent when compared with stereotactic higher doses.1,2 However, local control has evolved into a salient issue in recent years as improved systemic therapies has led to longer survival in cancer patients with metastatic disease. Prior to stereotactic body radiation therapy (SBRT), patients with good performance status frequently received doses up to 30 Gy/10 fractions.3 SBRT provides the capability of delivering precise high-dose radiation (biologic equivalent doses that are 2–3 times higher than the equivalent dose provided by 30 Gy/10 fractions) to oligometastatic disease, which may improve quality of life by extending the duration of pain control and delaying disease progression while reducing local side effects and the need for reirradiation.4,5 For radioresistant tumors such as melanoma and renal cell carcinoma, SBRT may offer improved local control with fewer late effects.6–8 For patients who have no symptoms at the time of SBRT delivery, the role of SBRT may be to defer initiation of systemic therapy by controlling local disease.
Multiple studies have been published on spinal and vertebral body SBRT as a salvage treatment for recurrent vertebral disease and cord compression.9–11 There are phase 2 trials under way to examine its role as first line treatment for cord compression and spinal bone metastases in a highly selected group of patients (Radiation Therapy Oncology Group 0631; Princess Margaret Hospital trial/MD Anderson Cancer Center trial). In contrast, there is a very little literature on the use of SBRT for non-spine bony metastases. A recent survey of radiation oncology practice in North America showed that SBRT is increasingly being adopted for the treatment of a number of oligometastatic sites including non-spine bony metastases12; yet the optimal dose and late effects such as fracture risk and osteoradionecrosis remain unknown.
The current study examines the Mayo Clinic experience treating patients with SBRT to non-spine bony metastasis.
Methods and materials
The Mayo Clinic has prospectively assessed, treated, and followed 74 patients from January 1, 2008 to August 1, 2012 with SBRT for non-spine bony metastases. Information was collected on patient age, sex, histology, bony site treated, pain relief, number of metastases at simulation, whether the treated site had previously received radiation therapy, local control, distant progression, radiographic response to treatment, SBRT prescription dose, chemotherapy delivery, and acute and late toxicity. Descriptive statistics were performed using JUMP (version 9.01; SAS Institute Inc, Cary, NC). Median progression-free survival, overall survival, and follow-up from the end of SBRT treatment were also calculated. Progression-free survival was defined as any progression (local or distant) from the end of SBRT treatment. Local failure was defined as in-field progression over serial imaging with computed tomographic (CT) scan, magnetic resonance imaging (MRI), and when available, positron emission tomography (PET)-CT. This study was approved by the Mayo Clinic institutional review board ethics board. Local control was defined as stable disease, partial response, or complete response based on serial imaging with CT scan, MRI, or PET-CT. A complete response was coded if there was complete disappearance of [18F]fluoro-2-deoxy-2-d-glucose-avidity on PET-CT or complete resolution of the tumor on CT scan or MRI.
Patients were immobilized using a 5 point mask for lesions above the T3 vertebral level and the commercially available BodyFix system (Electa AB, Stockholm, Sweden) for lesions below the T3 vertebral level. While we did perform 4-dimensional (4D)CT for some rib lesions, our experience was that the internal target volume did not change appreciably from the gross tumor volume (GTV) so this was not routinely performed. 4DCT was required for sternal lesions as there was significant anterior–posterior movement with respiration, although breath hold or gating was not routinely used for these lesions. Radiation therapy was delivered on a daily basis for fractionated regimens.
The SBRT plans were designed using Eclipse (Varian, Palo Alto, CA) treatment planning software. Generally, most patients had intensity modulated RT or volumetric modulated arc therapy techniques used to treat their bone lesions. The GTV was defined as the gross visible lesion on diagnostic PET-CT, CT scan, or MRI. The clinical target volume (CTV) encompassed the GTV plus 1 cm of contiguous bone and soft tissue extension if present. The planning target volume (PTV) included the CTV plus a 2-mm margin. The GTV was then expanded by 0 mm to be a high-dose PTV (range of doses, 16–24 Gy) and a low-dose PTV (range of doses, 14–18 Gy) was generated by expanding the CTV as defined previously. The dose was prescribed to cover the PTV by the 95% isodose line. Depending on location, adjacent normal tissue organs at risk were defined and kept below dose constraints as reported in TG101.13
Imaging was performed with the ExacTRAC 6D x-ray system (Brainlab, Felkirchen, Germany) with the 6D robotic couch. Corrections were applied and full verification imaging using both tube detector pairs was repeated to confirm positioning within 1–2 mm and 1 degree. Before delivering each treatment field, a “Snap” verification image using a single tube detector pair was acquired. Shifting occurred if the Snap verification image was greater than 2 mm. If necessary, a pair of kV orthogonal images or cone-beam CT was obtained to verify the isocenter.
While data were available in a prospectively collected Mayo Clinic SBRT database, all data were verified by retrospective chart review. Acute and late toxicity data were documented at every follow-up in a prospective manner. Additional information was gleaned from follow-up notes and notes documenting effects during the treatment course. The standardized Common Toxicity Criteria for Adverse Events, version 4.03, scale was applied retrospectively to these documented effects.
Results
Patient demographics
A total of 85 non-spine bone sites were treated with SBRT in 74 patients during the study period (Table 1). Most patients were female (48/74; 65%) but the predominant histology treated was prostate (23/74; 31%). The most common non-spine bone sites treated were the ilium and sacrum (41/85; 48%). Most patients were asymptomatic at the time of treatment (49/85; 58%) and most had oligometastatic disease (fewer than 5 sites of metastatic disease; 58/85; 68%). The goal of treatment for asymptomatic patients was local control and deferral of systemic treatment. Of the patients who had pain at the time of simulation (36 patients), 88% experienced subjective improvement in their pain. The median pain score of the 36 patients before treatment was 4.5 out of 10 (range, 1–10). Post-SBRT, 5 patients continued to have pain scores ranging from 2 to 3 (out of 10). One quarter of patients had previous radiation therapy to the SBRT site treated (20/85; 24%).
Table 1.
Demographics of stereotactic body radiation therapy (SBRT) non-spine bone metastases
| Variable | No. |
|---|---|
| Sex | (n = 74) |
| Male | 26 |
| Female | 48 |
| Median age at SBRT treatment | 60 (18–87 range) |
| Histology | (n = 74) |
| Breast | 6 |
| Prostate | 23 |
| Colon | 3 |
| Lung | 3 |
| Cervix | 1 |
| Endometrial | 1 |
| Head and neck | 5 |
| Melanoma | 6 |
| Sarcoma | 12 |
| Renal cell carcinoma | 5 |
| Paraganglioma | 1 |
| Thyroid | 2 |
| Carcinoid | 1 |
| Hepatocellular cancer | 1 |
| Endocrine | 1 |
| Testis | 1 |
| Bladder cancer | 1 |
| Unknown primary | 1 |
| Site treated | (n = 85) |
| Acetabulum | 3 |
| Chest wall | 1 |
| Clavicle | 1 |
| Coccyx | 2 |
| Femur | 2 |
| Ilium | 18 |
| Ischium | 7 |
| Mandible | 1 |
| Pelvis | 8 |
| Radial head | 1 |
| Rib | 10 |
| Sacrum | 23 |
| Scapula | 3 |
| Shoulder | 1 |
| Sphenoid sinus | 1 |
| Sternum | 2 |
| Trochanter | 1 |
| Number of metastases at simulation | (n = 85) |
| 1 | 36 |
| 2 | 7 |
| 3 | 11 |
| 4 | 2 |
| 5 | 2 |
| 6 | 2 |
| >10 | 25 |
| Prescription dose (per fraction, #) | (n = 85) |
| 15 Gy/1# | 1 |
| 16 Gy/1# | 3 |
| 18 Gy/1# | 15 |
| 20 Gy/1# | 4 |
| 21 Gy/1# | 3 |
| 21 Gy/3# | 1 |
| 22 Gy/1# | 1 |
| 24 Gy/1# | 16 |
| 24 Gy/3# | 8 |
| 27 Gy/3# | 1 |
| 30 Gy/3# | 10 |
| 30 Gy/5# | 1 |
| 35 Gy/5# | 1 |
| 36 Gy/3# | 2 |
| 40 Gy/5# | 8 |
| 45 Gy/5# | 2 |
| 50 Gy/5# | 8 |
Dose
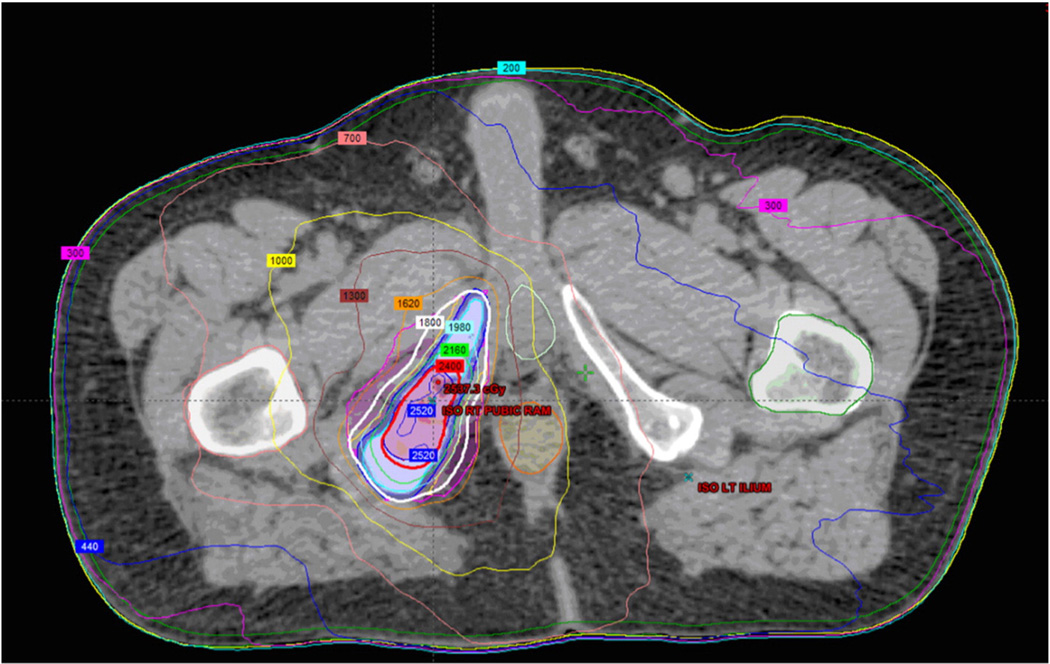
Dose prescriptions ranged from 15 Gy/1 fraction to 50 Gy/5 fractions. The most common dose prescriptions were 18 Gy/1 fraction, 24 Gy/1 fraction, and 30 Gy/3 fractions. The dose prescriptions used were extrapolated from our published institutional spine SBRT dose prescriptions.14 Most prostate adenocarcinoma patients were treated with 16 Gy/1 fraction based on our published institutional experience of 100% local control at 2 years in treating various oligometastatic prostate sites, including lymph nodes, liver, and spine.15 The mean PTV dose was 40 Gy with a median of 24 Gy/1 fraction. The mean biological effective dose (BED) delivered was 62.4 Gy10 and the mean single fraction equivalent dose (SFED) was 30.3 Gy. The mean PTV volume was 74 cc with a median of 25 cc (range, 2.7 cc–1030.8 cc). A sample radiation plan can be seen in Fig 1.
Figure 1.
Axial slice of stereotactic body radiation therapy (SBRT) bone plan. Red corresponds to the 24 Gy line and blue corresponds to the 18 Gy line. SBRT plan of the left ischium.
Acute toxicity
Most patients had no acute effects from SBRT treatment for their non-spine bony metastases. The characteristics of the 18 patients with documented acute toxicity are listed in Table 2. The most common acute toxicity was fatigue followed by an acute pain flare. There were no grade 3 or 4 toxicities. Most occurred immediately following the treatment. All patients were seen during the week of their radiation therapy treatments. Follow-up in the period after SBRT ranged between 1 and 3 months and were mostly with the patient’s medical oncologist. However, the database was prospectively updated with possible toxicity from the notes in the chart by a dedicated SBRT nurse. Of the 18 patients who experienced acute toxicity, 39% (7/18) had previous radiation therapy to their SBRT site. Of the dosimetric parameters, the number of fractions was associated with the development of early toxicity (P = .03) although PTV volume, SFED, and BED were not.
Table 2.
Acute toxicity in individual patients
| Case | Sex | Histology | Site treated | Dose | Retreat | Previous EBRT dose |
Time to toxicity |
Grade + toxicity |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | Cervix SCC | Coccyx | 40 Gy/5# | Yes | 50 Gy/25# | 7.5 d | Grade 1 pain flare |
| 2 | Female | Breast ca | Rt sacrum | 24 Gy/3# | No | 1 d | Grade 2 pain flare | |
| 3 | Male | Sarcoma | Pelvis | 40 Gy/5# | No | 5 d | Grade 1 pain flare | |
| 4 | Female | RCC | Rib | 50 Gy/5# | No | 1 d | Grade 1 fatigue | |
| 5 | Female | Lung ca | Rt ilium | 30 Gy/3# | No | 1 d | Grade 1 fatigue | |
| 6 | Female | Lung ca | Rt sacrum | 18 Gy/1# | No | 1 d | Grade 1 pain flare | |
| 7 | Male | HNSCC | Rib | 24 Gy/3# | No | 1 d | Grade 2 pain flare | |
| 8 | Male | RCC | Lt sacrum | 24 Gy/1# | No | 1 d | Dermatitis | |
| 9 | Male | Sarcoma | Rib | 24 Gy/1# | No | 2 d | Grade 3 pain flare | |
| 10 | Female | Endometrial | Acetabulum | 40 Gy/5# | Yes | 30 Gy/10# | 2 d | Grade 2 pain flare |
| 11 | Female | Endometrial | Lt S1 nerve root | 35 Gy/5# | Yes | 30 Gy/10# | 1 d | Grade 1 fatigue |
| 12 | Female | Melanoma | Sternum | 50 Gy/5# | No | 1 d | Grade 1 fatigue | |
| 13 | Female | Melanoma | Sacrum | 40 Gy/5# | Yes | 25 Gy/5# | 2 d | Grade 1 fatigue |
| 14 | Male | Colon ca | Rib | 24 Gy/3# | Yes | 20 Gy/5# | 4 d | Grade 1 fatigue |
| 15 | Female | Adenoca | Rt pelvis | 30 Gy/3# | Yes | 30 Gy/10# | 1 d | Grade 1 nausea |
| 16 | Female | Sarcoma | Lt sacrum | 45 Gy/5# | No | 2 d | Grade 2 pain flare + grade 1 nausea | |
| 17 | Male | Skin SCC | Rt shoulder | 15 Gy/1# | Yes | 60 Gy/30# | 1 d | Grade 1 fatigue |
| 18 | Male | Melanoma | Lt trochanter | 20 Gy/1# | No | 2 d | Grade 1 nausea |
#, fraction; Adenoca, adenocarcinoma; ca, cancer; EBRT, external beam radiation therapy; HNSCC, head and neck squamous cell carcinoma; Lt, left; RCC, renal cell carcinoma; Rt, right; SCC, squamous cell carcinoma.
Late toxicity
Median follow-up from the end of SBRT was 7.6 months (range, 0.1–41 months). Nine patients developed late effects (defined as sequelae greater than 90 days from the end of treatment) but none were more than grade 2 (Table 3). Two patients had fracture documented more than 90 days after treatment but both were asymptomatic. The fractures occurred in a sacral lesion and a clavicular lesion; neither patient had previous external beam radiation to those sites. The PTV volume, maximum dose to PTV, BED, SFED, and number of fractions had no predictive effect for late toxicity.
Table 3.
Demographics of late effects stereotactic body radiation therapy non-spine bone metastases
| Case | Sex | Histology | Site treated | Dose (#, fraction) |
Retreat | Previous EBRT dose |
Time to toxicity (days) |
Grade + toxicity |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | Adenoid cystic | Sacrum | 24 Gy/3# | No | 1045 d | Grade 1 Fracture (asymptomatic) |
|
| 2 | Female | Endometrial | S1 nerve root, left | 35 Gy/5# | Yes | 30 Gy/10# | 98 d | Grade 2 lymphedema |
| 3 | Male | Prostate | Rib, right | 18 Gy/1# | No | 58 d | Grade 1 Pulmonary fibrosis |
|
| 4 | Female | Renal cell carcinoma | Fifth right rib | 50 Gy/5# | No | 130 d | Grade 1 lymphedema | |
| 5 | Female | Endometrial | Acetabulum, left | 40 Gy/5# | Yes | 30 Gy/10# | 100 d | Grade 2 neuralgia |
| 6 | Male | Colon | Right posterior 8th rib | 24 Gy/3# | Yes | 20 Gy/5# | 238 d | Grade 2 chest wall pain |
| 7 | Male | Prostate | Left 8th rib | 18 Gy/1# | No | 172 d | Grade 2 pneumonitis/dyspnea | |
| 8 | Female | Carcinoid | Clavicle | 40 Gy/5# | No | 104 d | Grade 1 fracture (asymptomatic) | |
| 9 | Female | Osteosarcoma | Right iliac wing | 50 Gy/5# | No | 100 d | Grade 2 neuralgia |
EBRT, external beam radiation therapy.
Survival
Median SBRT specific overall survival was 9.25 months (range, 0.2–41 months) and median progression-free survival from the end of SBRT was 9.71 months (range, 0.1–38 months). Overall survival and progression-free survival from the end of SBRT treatment was 81.4% and 31.5%, respectively, at 1 year. Radioresistant histology predicted for poorer median progression-free survival (12.1 months vs 5.5 months; P < .0002). The number of metastases at simulation (<5 vs >5 metastases) was associated with reduced median overall survival post SBRT (10.8 months vs 6.4 months; P < .0001) but had no impact on median progression-free survival (P =.08).
Local control
Local control at 12 months was 91.8%. The median time to local recurrence was 2.8 months (range, 1.9–21.6 months). None of the patients who experienced local failure had prostate adenocarcinoma histology. Of the 7 patients with in-field recurrence, the histologies included cervical cancer, papillary thyroid cancer, renal cell carcinoma, dedifferentiated liposarcoma, melanoma, and hepatocellular carcinoma. Radioresistant histology did predict for local failure (P = .03) and high risk of distant metastases (P < .001). The bony sites that failed locally were heterogeneous and lay within the coccyx, sacrum, scapula, and rib. Only 1 of the 7 patients who failed locally had previous radiation therapy to the SBRT site (previous 50 Gy/25 fractions). The doses prescribed to the sites were heterogeneous as well and ranged from 24 Gy/1 fraction to 40 Gy/5 fractions. The number of metastases at simulation had no impact on local control. No dosimetric parameters were associated with local failure.
Discussion
The current study is the largest series examining the use of SBRT for non-spine bony metastases in a heterogeneous group of patients. Previous reports have looked at the role of SBRT in specific radioresistant histologies, most notably renal cell carcinoma and melanoma. In a series from Memorial Sloan-Kettering (New York, NY), 105 extracranial renal cell carcinoma lesions were treated predominantly with a single SBRT fraction (24 Gy) with a 3-year local control rate of 88%. Fractionation schedules of 20–30 Gy in 3–5 fractions, or single fractions <24 Gy, showed vastly inferior local control rates of <25%.7 Pain control for renal cell carcinoma metastases is also much improved with SBRT compared with conventional radiation, especially if the BED was greater than 85 Gy.8 In our series, 88% of patients who presented with bony pain had complete resolution of their pain after SBRT treatment. In one series examining melanoma patients, 17 patients with 28 lesions were treated with SBRT to doses of 40–50 Gy/5 fractions or 40–60 Gy/3 fractions. Local control was 88% at 18 months for all patients.6 A recent dose escalation study for patients with heterogeneous histologies also supports that higher BED is correlated with local control. In this study, patients received between 24 Gy/3 fractions and 48 Gy/3 fractions to 1–5 sites of oligometastatic disease. Local control at 2 years was 88.2% for patients treated with 30 Gy, 36 Gy, and 42 Gy in 3 fractions but only 46%for those treated with 24 Gy/3 fractions.16 Our series shows excellent local control with 91.8% local control at 1 year and 86% at 2 years. The 7 patients who failed locally in our study likely had very aggressive disease given that they recurred in-field within the first 12 months of treatment. Most of these patients had radioresistant histologies.
In cancer histologies (where patients typically have a long median survival) such as breast and prostate cancer, prolonged local control may allow patients to defer systemic therapy. A study from the University of Rochester Medical Center (Rochester, NY)17 of breast cancer patients who received SBRT for oligometastatic disease showed a 2 year local control rate of 87% and overall survival of 74%. The same study found that patients with non-breast histologies, predominantly lung cancer, fared much worse due to a short disease-free and progression-free interval. Recently, a phase 2 trial of SBRT in metastatic prostate cancer was conducted in men with oligometastatic disease as a means to delay androgen deprivation therapy.18 Their SBRT dose was 50 Gy/10 fractions conferring 100% 2-year local control and a median hormone therapy avoidance period of 38 months. A recent trial by Salama et al16 examined the role of SBRT for patients with oligometastatic disease of any histology who had a life expectancy greater than 3 months. Sixty-one patients were enrolled in the trial and a number of sites were treated including 15 osseous metastases. With a median follow-up of 20.9 months, progression-free survival and overall survival were 33.3% and 81.5% at 1 year.16 The reported progression-free survival and overall survival in our series is in keeping with that reported in the literature. We have also published our institutional experience treating prostate cancer oligometastases with 16 Gy/1 fraction with a 100% local control rate at 2 years.15 Given the excellent overall survival of many patients with oligometastatic disease, SBRT may be a reasonable complement to systemic therapies in patients with cancer whose histologies are less prone to rapid distant failure.
Our study confirms that SBRT for non-spine bone metastases is very tolerable. Acute effects were predominantly limited to pain flare and fatigue. Even with conventional radiation therapy, there is up to a 33% chance of a pain flare.19,20 In the current series, only 10%of patients experienced a pain flare. This is reassuring given that more than half the patients we treated had no symptoms prior to SBRT. Fatigue was also quite common although the duration of fatigue was not well documented in the chart. No grade 3 or 4 toxicities were reported despite the location of most treatment fields and volumes in the pelvis which often lies adjacent to bowel, bladder, and sacral plexus.
Only 9 patients in our series developed late effects. Interestingly, 4 of 9were associated with rib treatments with 2 patients developing radiation pneumonitis in the adjacent lung and another 2 with neuralgia and lymphedema. The lung SBRT literature notes very low incidences of chest wall pain with BED <100 Gy in a single dose.21 However, follow-up beyond 16 months showed up to a 39% incidence of radiation-induced chest wall pain.22 There is some suggestion that obesity may be predictive of chest wall toxicity in lung SBRT.23,24 None of the rib patients who were treated with SBRT in our series developed fractures. The 2 fractures noted were asymptomatic (clavicle and sacrum). For SBRT of other sites, including lung and spine, the late fracture risk has been reported to be 10% to 20% with most being asymptomatic.25
While this study represents the largest single institution series on non-spine bone metastases, it is limited by a short median follow-up (7.6 months). Documentation of side effects of radiation therapy was challenging as patients were inconsistently followed up after receiving a single fraction of radiation. This study is also limited by retrospective chart review and physician reported toxicity.
It is likely that the use of SBRT for non-spine bone metastasis will increase with improved systemic control of multiple tumor sites. In addition to local control, SBRT may offer long-term pain relief where conventional radiation therapy may fail. The American College of Radiology recently published a survey of the appropriateness of this technology in this setting.26 While the study largely concentrated on the use of EBRT, a number of respondents did indicate that they would offer SBRT. With wider use of SBRT, a key issue will be the selection of appropriate candidates. In recently published reports and in the current study, more than half of patients treated go on to progress at distant sites. Overall survival from the end of SBRT varies significantly with histology, the burden, and pace of disease. In the end, SBRT for non-spine bone metastases may be best for patients who have good systemic control and isolated disease that requires long-term local control.
Footnotes
Conflicts of interest: None.
References
- 1.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 2.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75:1501–1510. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 4.Jhaveri P, Teh BS, Bloch C, Amato R, Butler EB, Paulino AC. Stereotactic body radiotherapy in the management of painful bone metastases. Oncology (Williston Park) 2008;22:782–789. [PubMed] [Google Scholar]
- 5.Lutz S, Lo SS, Chow E, Sahgal A, Hoskin P. Radiotherapy for metastatic bone disease: current standards and future prospectus. Expert Rev Anticancer Ther. 2010;10:683–695. doi: 10.1586/era.10.32. [DOI] [PubMed] [Google Scholar]
- 6.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol. 2012;51:584–588. doi: 10.3109/0284186X.2011.652741. [DOI] [PubMed] [Google Scholar]
- 9.Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652–665. doi: 10.1016/j.ijrobp.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JW, Yoo DS, Sampson JH, et al. Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys. 2009;73:1369–1375. doi: 10.1016/j.ijrobp.2008.06.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 12.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed KA, Stauder MC, Miller RC, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:e803–e809. doi: 10.1016/j.ijrobp.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed KA, Barney BM, Davis BJ, Park SS, Kwon ED, Olivier KR. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2012;2:215. doi: 10.3389/fonc.2012.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salama JK, Hasselle MD, Chmura SJ, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118:2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 17.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Berkovic P, De Meerleer G, Delrue L, et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11:27–32. doi: 10.1016/j.clgc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Hird A, Zhang L, Holt T, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol) 2009;21:329–335. doi: 10.1016/j.clon.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy–a nested randomized control trial. Support Care Cancer. 2007;15:451–455. doi: 10.1007/s00520-006-0166-y. [DOI] [PubMed] [Google Scholar]
- 21.Trakul N, Chang CN, Harris J, et al. Tumor volume-adapted dosing in stereotactic ablative radiotherapy of lung tumors. Int J Radiat Oncol Biol Phys. 2012;84:231–237. doi: 10.1016/j.ijrobp.2011.10.071. [DOI] [PubMed] [Google Scholar]
- 22.Mutter RW, Liu F, Abreu A, Yorke E, Jackson A, Rosenzweig KE. Dose-volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:1783–1790. doi: 10.1016/j.ijrobp.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsh J, Thomas J, Shah D, et al. Obesity increases the risk of chest wall pain from thoracic stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:91–96. doi: 10.1016/j.ijrobp.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J Neurosurg Spine. 2012;16:379–386. doi: 10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]
- 25.Cunha MV, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84:e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spine bone metastases. J Palliat Med. 2012;15:521–526. doi: 10.1089/jpm.2011.0512. [DOI] [PubMed] [Google Scholar]