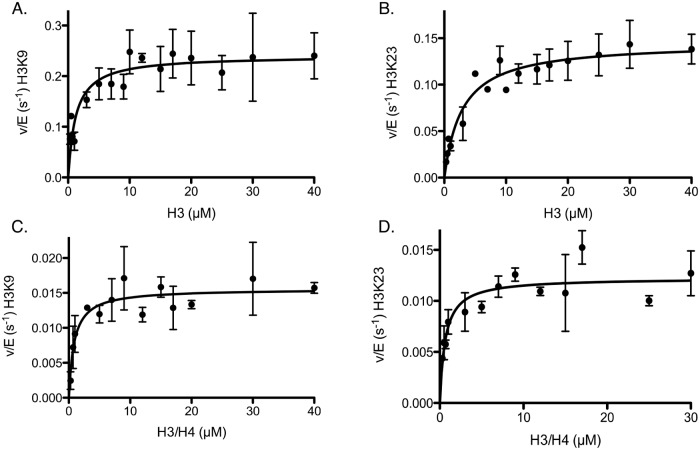
Fig 2. Determination of steady-state parameters for individual lysines of H3 and H3/H4 acetylation catalyzed by Rtt109-Vps75 when titrating histone in the presence of saturating acetyl-CoA.
The detailed experimental conditions are described in the section “Steady-state kinetic assays for Rtt109-Vps75”. The error bar represents the standard error in v/E. Panels (A) and (B) show the Michaelis-Menten behaviors for H3K9 and H3K23, respectively, when titrating H3. Panels (C) and (D) also show the Michaelis-Menten behaviors for K9 and K23, respectively, when titrating H3/H4. H3 is the preferred substrate for Rtt109-Vps75 acetylation. The apparent kinetic parameters are summarized in Table 1.