Table 1.
Potency of adenosine analogues at a coronary A2-adenosine receptor and at a brain A1-receptor
| Analogue | A2 Receptor |
A1 Receptor | EC50(A2) |
|
|---|---|---|---|---|
| MPRa Relative to Ado |
Estimated Potencyb EC50(nM) |
Ki(nM)c | Ki(A1) | |
| 1 NECA | 150 | 8 | 5.1 | 1.6 |
| 2 2-chloroadenosine | 27 | 44 | 6.7 | 6.6 |
| 3N6-methyladenosine | 0.05 | 24,000 | 60 | 400 |
| 4 R-PIA | 4.3 | 280 | 1.2 | 230 |
| 5 CHA | 1.6 | 750 | 0.85 | 880 |
| 6 N6-phenyladenosine | 1.4 | 860 | 3.3 | 260 |
| 7 N6-p-tolyladenosine | 1.35 | 890 | 2.5 | 360 |
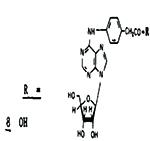
|
[27% @ 21μM]d | 210 | - | |
| 9 NHCH3 | 4.9±1.3 | 240 | 16 | 15 |
|
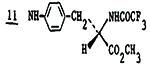
|
2.1±1.3 | 570 | 1.7 | 340 |
|
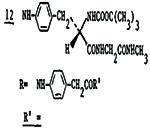
3.0±0.7 | 400 | 18 | 22 |
|
4.4±0.4 | 270 | 13 | 21 |
| 13 OCH3 | 3.2±1.6 | 380 | 2.5 | 150 |
| 14 NHCH3 | 7.2±1.3 | 170 | 6.7 | 25 |
| 15 NHNH2 | 3.5±0.8 | 340 | 4.5 | 80 |
| 16 NH(CH2)2NH2 | 7.8±2.5 | 150 | 0.85 | 180 |
|
|
2.8±0.1 | 430 | 4.5 | 96 |
| 18 NH(CH2)2NH-CO–biotin | 3.0±0.2 | 400 | 11.4 | 35 |
| 19 NH(CH2)2NHCO(CH2)5NH-CO–biotin | 10.2±7.3 | 120 | 18 | 6.7 |
Molar potency ratio relative to adenosine, which is set equal to 1.0.
Estimated IC50 values based on potency of adenosine (MPR = 1.0) of 1,200±150 nM (6) .
Ki values for antagonism of binding of 1nM [3H]N6-cyclohexyladenosine to rat cerebral cortical membranes (data from 3, 4, 12).
Highest concentration of analogue did not raise coronary blood flow to a level × 50% of maximum possible increase. In such a case we report % increase in flow over control at the plasma nucleoside concentration achieved during infusion.
