Figure 1.
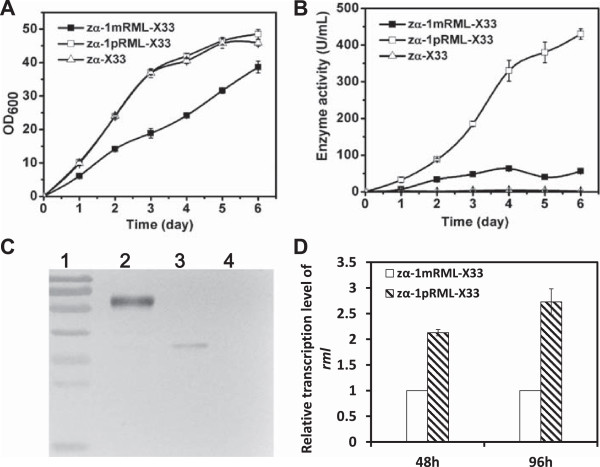
Effect of propeptide addition on cell growth and target enzyme activity. A: OD600 of recombinant strains in flask fermentation. Cell growth of control strain (zα-X33) and recombinant strain (zα-1pRML-X33) containing the propeptide sequence was much higher than that of the recombinant strain without the propeptide sequence (zα-1mRML-X33). B: Enzyme activity of recombinant strains in flask fermentation. Extracellular enzyme activity of zα-1pRML-X33 (430 U/mL) was 7.7-fold higher than that of zα-1mRML-X33 (56 U/mL). C: Extracellular protein production in fermentation supernatant detected by Western blotting. Lane 1: protein markers (top to bottom: 100, 70, 55, 40, 35, 25, 15 kDa). Lane 2: zα-1pRML-X33. Lane 3: zα-1mRML-X33. Lane 4: zα-X33. Each sample tested was 10 μL of 10 × diluted fermentation supernatant. The concentration of secreted extracellular target protein for zα-1pRML-X33 (0.15 mg/mL) was higher than for zα-1mRML-X33 (0.019 mg/mL). D: Comparison of the transcription level (by qPCR) of rml in zα-1pRML-X33 vs. zα-1mRML-X33. When the same signal peptide codons were present in the expression plasmid, adding the propeptide resulted in upregulation of target gene expression at both 48 and 96 hours.
