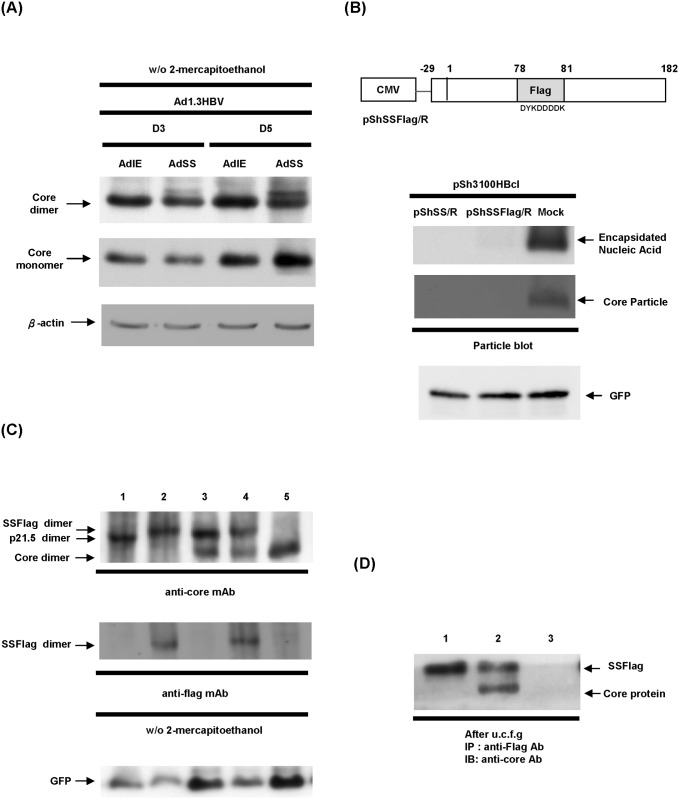
Fig 3. The p21.5 viral protein forms homodimers that assemble with core protein dimers.
(A) HepG2 cells were co-transduced with Ad1.3HBV together with either AdSS or AdIE and total cell lysates were harvested at day 3 and day 5 post-transduction. SDS-PAGE was performed on the samples in the absence of 2-mercaptoethanol. The expression levels of core protein monomer and dimer were revealed by western blot analysis using an anti-core antibody. (B) Upper panel, schematic of the coding structure of pShSSFlag/R in which the amino acids 78th-81th (DPAS) of the core ORF were replaced by the flag sequence (DYKDDDDK). Lower panel, 293T cells were co-transfected with the indicated plasmids and the expression of intracellular nucleocapsids was determined by particle blot analysis. (C) 293T cells were co-transfected with the indicated plasmids for 3 days, and the cell lysates were harvested and SDS-PAGE was performed without 2-mercaptoethanol. The expression levels of core protein monomer and dimer were examined by western blot analysis using anti-core antibody and anti-FLAG antibody. The GFP signal was used as a loading control. Lane 1: pShSS/R; lane 2: pShSSFlag/R; lane 3: pSh3100HBcl and pShSS/R; lane 4: pSh3100HBcl and pShSSFlag/R; lane 5: pSh3100HBcl and pShuttle/R(Mock). (D) 293T cells were co-transfected with the indicated plasmids for 3 days, and then the cell lysates were harvested and pelleted by sucrose cushion centrifugation. The assembled larger viral components were immnoprecipitated by anti-FLAG antibody, followed by immunoblotting with anti-core antibody. Lane 1: pShSSFlag/R and pShuttle/R; lane 2: pSh3100HBcl and pShSSFlag/R; lane 3: pSh3100HBcl and pShuttle/R.