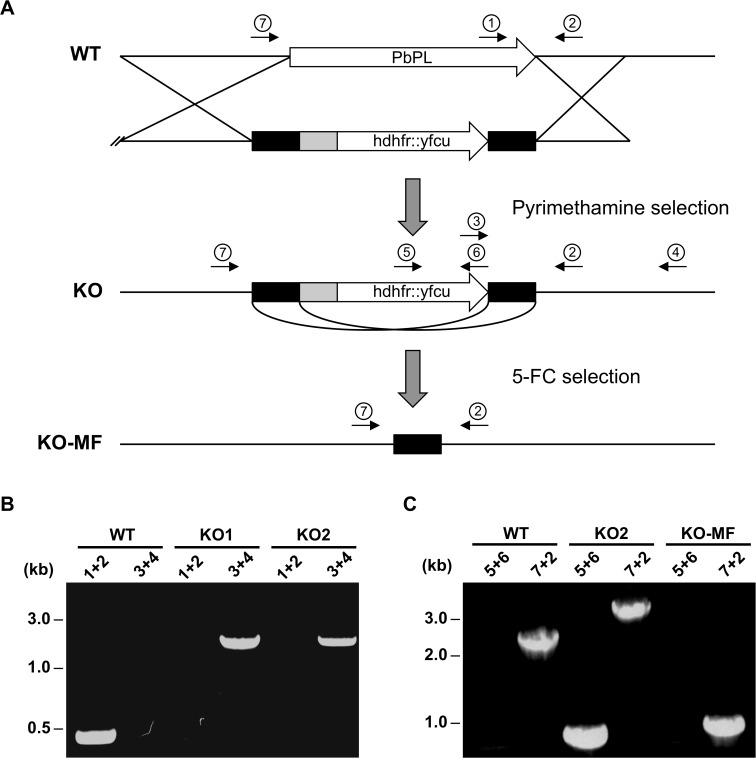
Fig 2. Generation and genotype analyses of PbPL-knockout parasite lines.
A) Schematic representation of knockout (KO) strategy and marker recycling. Two clonal PbPL-KO parasite lines were generated by transfection of wild-type (WT) blood stage parasites with a plasmoGEM vector containing a fusion of the positive drug selectable marker hdhfr (human dihydrofolate reductase) and the negative marker yfcu (yeast cytosine deaminase and uridyl phosphoribosyl transferase) under the control of the P. berghei eef1α promoter (grey box) targeting the PbPL coding sequence by double crossover homologous recombination followed by pyrimethamine selection. The selection marker was removed by negative selection with 5-Fluorocytosine (5-FC), whereby marker-free PbPL-KO parasites (KO-MF) were selected that had undergone homologous recombination between the two 3’dhfr untranslated regions (black boxes) present in the targeting vector flanking the hdhfr::yfcu cassette. Locations of primers used for PCR analysis are shown. B) Diagnostic PCR to confirm PbPL-KO clones. Primer 1 and 2 are expected to give a product of 398 bp in case of WT parasites, while primer 3 and 4 are expected to yield a product of 2058 bp for KO parasites. C) Diagnostic PCR to confirm successful removal of selectable marker. Primers 5 and 6 bind in the yfcu gene and are therefore expected to only give a product of 909 bp in case of selectable marker containing KO parasites. Primers 7 and 2 are expected to give a product of 2568 bp in case of WT, a product of 3782 bp for KO and a product of 1001 bp for KO-MF parasites. All primer sequences are listed in S1 Table.