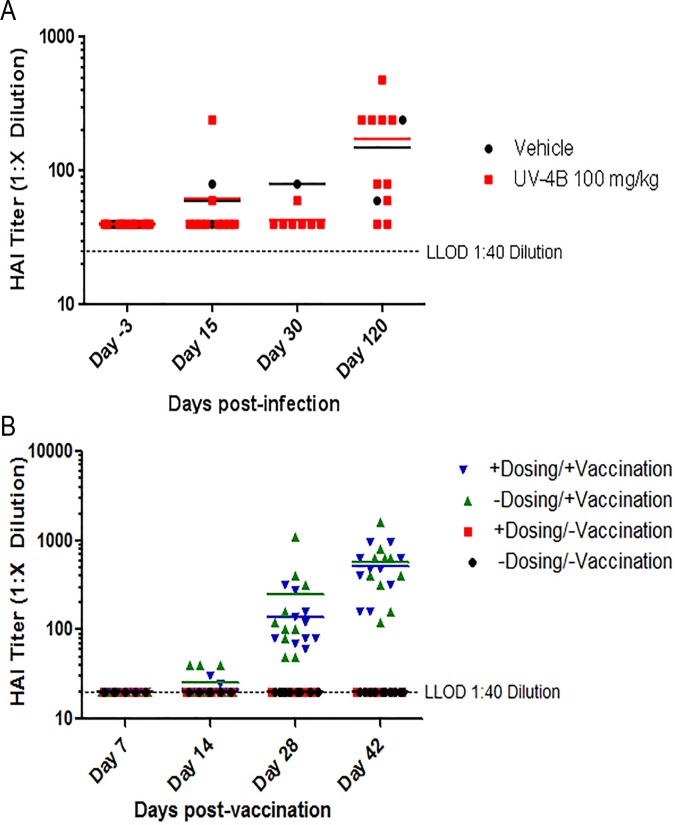
Fig 5. Influence of UV-4 on antibody titers induced by influenza infection or vaccination.
(A) Groups of mice received the first treatment dose of compound (n = 10) or water (n = 20) starting 1 hour before an IN infection with INFV. Serum samples were collected on days-3, 15, 30, and 120 post-infection. Samples were treated with receptor-destroying enzyme II before being added to chicken red blood cells for determination of HAI activity. (B) Mice were split into 4 groups of 10 mice either vaccinated with or without UV-4 treatment or no vaccination with or without UV-4 treatment; where relevant, mice were dosed orally with 100 mg/kg of UV-4, TID, for 10 days total. Mice receiving vaccine were injected on days 0, 14, and 28 with 50 μL of Fluvirin 2010/2011 (Novartis, containing A/California/07/2009, A/Perth/16/2009, and B/Brisbane/60/2008) vaccine intramuscularly. Mice were bled on days 0, 14, 28 and 42 and serum of mice was tested for inhibition of hemagglutination against homologous seasonal flu vaccine Fluvirin 2010–2011.