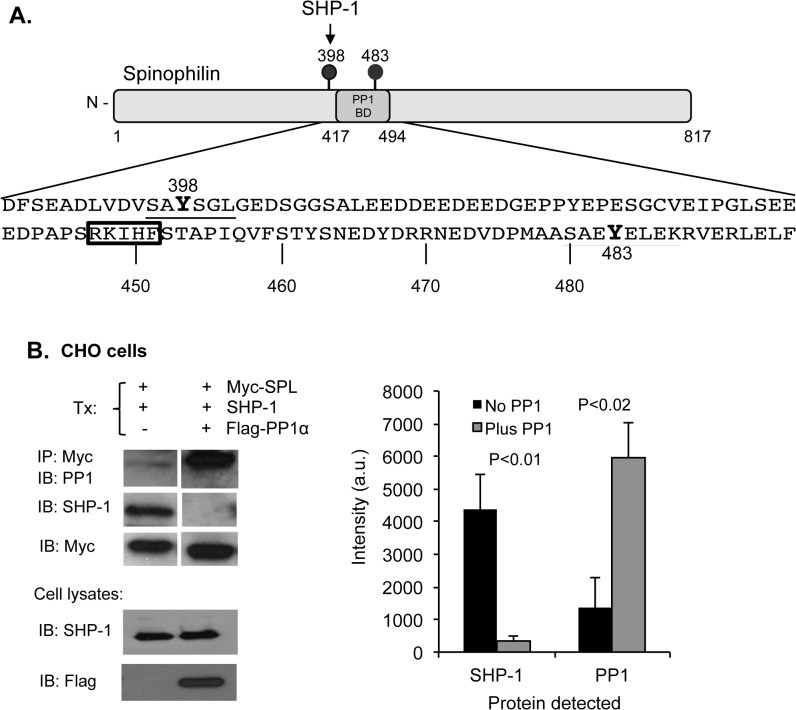
Fig 3. Competition of PP1 and SHP-1 for spinophilin.
(A) A diagram of spinophilin showing the PP1 binding domain [31] and the SHP-1 binding site on phosphorylated Y398 [2]. There are two tyrosine phosphorylated residues in spinophilin: Y398 and Y483. The boxed RKIHF sequence matches a consensus sequence for PP1 binding (R/K)(R/K)(V/I)x(F/W) [27]. (B) Top: CHO cells were transfected with Myc-SPL, SHP-1 and Flag-PP1α as indicated. Proteins were precipitated with anti-Myc and probed for either SHP-1 or PP1. Bottom: Western blots of transfected cell lysates probed for SHP-1 or Flag-PP1α. The graph shows the amount of SHP-1 (left columns) or PP1 (right columns) that co-precipitated with SPL. For the black bars, only endogenous PP1 was present. For the gray bars, the cells were transfected with FLAG-PP1 (mean ± SEM, N = 5).