Abstract
Context:
Chronic periodontitis is an inflammatory condition of the tooth supporting structures. There is increasing evidence that the cytokines interleukin-17 (IL-17) and interleukin-18 (IL-18) play a role in progression of chronic periodontitis.
Aim:
The objective of this study was to compare the levels of the cytokines IL-17 and IL-18 in gingival tissue extracts from individuals with healthy gingiva, chronic gingivitis, and mild chronic periodontitis.
Settings and Design:
The study was performed in a hospital-based population with an experimental design.
Materials and Methods:
A total of 69 individuals (n = 23 per group) were recruited for the study. Group 1 included 23 individuals with healthy gingiva and Group 2 included 23 chronic gingivitis patients and Group 3 included 23 patients with mild chronic periodontitis. Gingival tissues were collected during surgical procedures and levels of IL-17 and IL-18 were determined using enzyme-linked immunosorbent assay.
Statistical Analysis:
Intergroup comparison was done by posthoc Tukey's test.
Results:
The gingival tissue concentration of IL-17 was found to be highest in Group 2 (415.19 ± 76.84 pg/mg) followed by Group 3 (193.77 ± 37.32 pg/mg) and Group 1 (20.49 ± 6.05 pg/mg). Concentrations of IL-18 were significantly higher (P < 0.01) in Group 2 (1479.42 ± 330.33 pg/mg) when compared with Group 1 (385.18 ± 71.26 pg/mg) and Group 3 (330.24 ± 48.56 pg/mg).
Conclusion:
There appears to be considerable variation of IL-17 and IL-18 levels in gingival tissue during periodontal health and disease.
Keywords: Chronic gingivitis, chronic periodontitis, gingiva, interleukin-17, interleukin-18
INTRODUCTION
Chronic periodontitis, an inflammatory condition of the tooth supporting structures occurs as a result of the complex interaction between periodontopathic bacteria and cells of host immune system. Resident and nonresident cells in the inflammation site are responsible for production of cytokines, which play a role in pathogenesis of periodontal disease.[1] The cellular immune response is characterized by infiltration of T cell into the periodontal tissues and differentiation into diverse subsets, which is influenced by the cytokine milieu.[2] The T cells subsets can be classified as helper T cells, cytotoxic T cells, and regulatory T cells. Among the T helper cell subsets, Th1 and Th2 cells have been most extensively researched upon. The Th1/Th2 balance is pivotal in immunoregulation of periodontal disease and is influenced by genetic factors, the characteristic of antigen (s), antigen presenting cell (APC), the immune response, and T cell receptor interactions.[3] It has been proposed that the stable lesion in periodontitis is mediated by Th1 cells, whereas progression of the lesion reflects a shift toward Th2 subset of cells.[4] The cytokines interleukin IL-12, IL-18, interferon gamma, and tumor necrosis factor alpha are involved in Th1 immune response and in contrast IL-4, IL-5, and IL-13 are involved in Th2 immune response and promoting humoral immunity.[5]
The “protective Th1/destructive Th2” model is disputed by some studies.[6,7,8] A novel subset of CD4 + T cells which explains many of the discrepancies in the classic Th1/Th2 model, has been identified and termed “Th17” based on the secretion of the cytokine IL-17. Cytokines characteristic of this subset have been found in inflamed periodontal tissue, suggesting their potential role in periodontal pathogenesis.[9,10,11,12] The presence of Th17 cells has been demonstrated in gingiva of patients with chronic periodontitis and there is an increasing evidence that Th17 cytokine plays a dominant role in progression of periodontal disease.[9]
Interleukin-18 a member of the IL-1 ligand superfamily is primarily produced by APCs,[13,14] and also by osteoblasts, adrenal cortex cells.[15] and oral epithelial cells.[16] It has been found to be up regulated in various chronic inflammatory diseases, including periodontal disease.[17,18] IL-18 could play a significant role in progression of periodontal disease because of its chemotactic, proinflammatory, and angiogenic properties and this cytokine also increases the rates of neutrophil activation.[17]
The objective of this study was to compare the levels of the cytokines IL-17 and IL-18 in gingival tissue extracts from individuals with healthy gingiva, chronic gingivitis, and mild chronic periodontitis.
MATERIALS AND METHODS
A total of 69 individuals in the age range of 24-55 years attending the outpatient Department of Periodontology, Faculty of Dental Sciences, Sri Ramachandra University, Chennai were recruited for this study after obtaining written informed consent between June 2012 and December 2012. The study was approved by Institutional Ethics Committee. The individuals were enrolled into three groups (n = 23 per group) based on specific inclusion and exclusion criteria.
Group 1 (controls) included 23 individuals with clinically healthy gingiva as determined by the absence of clinical signs of inflammation, presence of probing pocket depth ≤3 mm, absence of bleeding on probing, no clinical attachment loss, no mobility or furcation involvement and no radiographic evidence of bone loss. The inclusion criteria for Group 2 (chronic gingivitis) was hyperplastic inflammatory gingival enlargement with at least 10 natural teeth, Loe and Silness gingival index score > 1, presence of gingival color change, presence of bleeding on probing, presence of probing pocket depth 3-5 mm, no loss of attachment, no radiographic evidence of bone loss. Group 3 (chronic periodontitis) included 23 patients who were diagnosed to have generalized chronic periodontitis (Armitage et al., 1999):[19] Presence of at least 10 natural teeth, attachment loss ≥1 mm in >30% of the sites examined, abundant local factors, radiographic evidence of bone loss.
The exclusion criteria for all the groups are as follows: History of tobacco usage in any form, individuals who have taken antibiotics for past 6 months, individuals who have taken analgesics for past 1 week, pregnant and lactating women, presence of any other systemic disease, individuals who had undergone previous periodontal treatment.
Clinical examination
A thorough medical and dental history was elicited using a structured proforma. Plaque index (Silness and Loe, 1964)[20] and gingival index (Loe, 1967)[21] was recorded. Periodontal parameters assessed (on all the teeth excluding the third molars) included probing at six sites per tooth using an UNC 15 probe to determine probing pocket depth and clinical attachment level. Radiographic examination was done only for chronic periodontitis patients with the use of intraoral periapical radiographs (long cone technique).
Gingival tissue collection and processing
Following clinical examination, gingival tissue collection was done from individuals with healthy gingiva undergoing crown lengthening procedure/extraction for orthodontic purpose. Tissue samples from chronic gingivitis patients were collected during gingivectomy procedure to treat hyperplastic gingival enlargement. Chronic periodontitis tissue samples were collected from mild periodontitis sites with probing depth of ≥5 mm, attachment loss ≤2 mm, and prior to nonsurgical periodontal therapy. Gingival tissue processing was done as per the protocol given by Johnson et al.[9]
Ten milligram of gingival tissue was collected from healthy, gingivitis, and mild chronic periodontitis sites under local anesthesia (2% xylocaine, 1:200,000 adrenaline) and transferred to Eppendorf tubes and stored in minus 20°C. Tissues were weighed in a microbalance (to standardize the weight of the tissue samples) and completely solubilized by grinding with mortar and pestle in phosphate buffered saline (pH 7.4). Aliquots were stored in Eppendorf tubes at minus 20°C until use. Levels of IL-17 (Ray Biotech Inc., Norcross, GA, USA) and IL-18 (R and D Systems, Minneapolis, USA) were determined by the enzyme-linked immunosorbent assay (ELISA) method as per manufacturer's protocol. In brief, 100 μl of the samples were added (in duplicate) in the 96 well anti-human antibody (IL-17 and IL-18) coated plates and were incubated at room temperature for 2 h. The wells were washed with wash buffer after incubation. This was followed by addition of the secondary conjugate and incubation for 45 min at room temperature. Finally, 50 μl of the stop solution was added and the plates were read at 450 nm immediately using an ELISA plate reader.
Statistical analysis
Mean and standard deviation of the continuous variables were calculated. Intergroup comparison of the mean levels of IL-17 and IL-18 in gingival tissues was performed using a posthoc Tukey's test. Statistical analysis was performed using SPSS software version 16 IBM, Armonk, New York, USA. The difference in mean was considered statistically significant if P < 0.05.
RESULTS
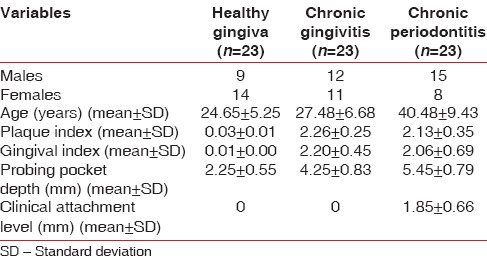
Descriptive and clinical data are presented in Table 1. Of 69 individuals, 36 were males and 33 were females. Mean gingival index scores were found to be higher amongst individuals in the chronic gingivitis group when compared with the chronic periodontitis and healthy group.
Table 1.
Descriptive data for the study participants
Interleukin-17 and interleukin-18 levels in different groups
The gingival tissue concentration of IL-17 was found to be highest in Group 2 (chronic gingivitis) followed by Group 3 (chronic periodontitis) and Group 1 (healthy) [Table 2]. A statistically significant difference in the mean concentration of IL-17 levels was observed between Groups 1 and 2 (P = 0.000) and between Groups 2 and 3 (P = 0.018). However, no statistical significance was observed between the Groups 1 and 3 (P = 0.080) [Table 3]. An intergroup comparison of mean gingival tissue concentration of IL-18 was done. Concentrations of IL-18 were significantly higher in Group 2 when compared to Groups 1 and 3. The difference in the mean concentration was statistically significant for Group 1 versus 2 (P = 0.001) and for Group 2 versus 3 (P = 0.000). No statistically significant difference was observed between Group 1 and 3 (P = 0.980) [Table 4].
Table 2.
Mean concentration of IL-17 and IL-18 in gingival tissue

Table 3.
Intergroup comparison of IL-17 levels by posthoc Tukey's test

Table 4.
Intergroup comparison of IL-18 levels by posthoc Tukey's test

DISCUSSION
The observations of our study provide evidence for the presence of IL-17 and IL-18 in detectable levels in the gingival tissue of individuals with healthy gingiva, patients with chronic gingivitis and chronic periodontitis. Our observations are in concurrence with those of Cardoso et al.[22] who demonstrated elevated levels of IL-17 mRNA and protein levels in gingiva of patients with chronic periodontitis. Similar observations have been made by Vernal et al.[11] in gingival crevicular fluid and in supernatants of cell cultures from periodontitis tissue by Takahashi et al.[10]
The results of this study contradict the observations of Pradeep et al.[23] who reported IL-17 levels could not be estimated in detectable levels in gingival crevicular fluid of individuals with healthy, patients with gingivitis and mild chronic periodontitis. This may possibly be due to the difference in the sample in which the IL-17 levels were assessed. In the present study, a higher level of IL-17 was observed in gingival biopsies from chronic gingivitis sites when compared to periodontitis and healthy sites. This observation is in concurrence with the findings of Oda et al.[24] who investigated the effect of outer membrane protein of the Porphyromonas gingivalis on IL-17 and RANKL expression in peripheral blood mononuclear cells (PBMC) from subjects with gingivitis and chronic periodontitis. The authors observed higher levels of IL-17 protein in PBMC culture supernatants from gingivitis samples as compared to chronic periodontitis samples although, no significant difference between the two groups could be established for mRNA levels.
A similar trend in the IL-17 levels has been observed by Johnson et al.[9] The authors assessed the levels of IL-11, IL-17, RANTES, and IL-6 in gingival biopsies from healthy sites (≤3 mm sulcus depth) and diseased gingiva (≥3 mm pocket with bleeding) and reported IL-17 concentrations were highest at 4-5 mm sites. In addition, the concentrations of the cytokines were reported to be lower in gingival biopsies adjacent to pocket ≥6 mm. These observations concur with our finding of high IL-17 levels in gingival tissue from chronic gingivitis as compared to chronic periodontitis tissue samples. Yu et al.[25] evaluated the role of IL-17 in inflammatory bone loss induced by the oral pathogen P. gingivalis in IL-17 RA deficient mice. The deficient mice exhibited alveolar bone destruction suggesting a bone protective role for IL-17, which was mediated through neutrophils. The observation in this study of a reduction of IL-17 levels in chronic periodontitis tissue samples appears to be in agreement with the findings of Yu et al.[25]
Interleukin-18, which was initially described as interferon gamma inducing factor, is now known as a cytokine that stimulates both Th1 and Th2 response depending on the presence or absence of IL-12.[14] In the presence of IL-12, IL-18 mainly induces the production of interferon gamma producing Th1 cells and in absence of IL-12, it shifts the response to IL-4 producing Th2 cells.[14] Hence, IL-18 appears to be a potential factor that can play a key role in regulating the immune responses involved in the initiation and progression of periodontal disease.
In our study, we assessed the levels of IL-18 in healthy, gingivitis and in chronic mild periodontitis sites and elevated levels of IL-18 were observed in gingivitis samples followed by healthy and periodontitis samples. Our study results are in conflict with previous study reports wherein levels of IL-18 have been found to increase with progression of disease from healthy to gingivitis to chronic periodontitis.[17,23,26] The increased levels of IL-18, in gingivitis sample when compared to periodontitis sample may be attributed to the difference in the degree of gingival inflammation and sample collection from mild sites in chronic periodontitis group. The mean gingival index was 2.203 for gingivitis group, whereas in periodontitis group, it was about 2.059. It has been reported that APCs[12] and epithelial cells[15] are the source of IL-18. APCs are present in larger numbers in gingivitis lesions when compared to periodontitis lesions.[27] This may explain the higher levels of IL-18 observed in chronic gingivitis when compared to chronic periodontitis group.
Interleukin-18 appears to have a pleiotropic activity as evidenced by the observations of Horwood et al.[28,29] and Udagawa et al.,[30] wherein IL-18 acts as an inhibitor of osteoclast formation through indirect effects mediated by T cells production of granulocyte macrophage colony stimulating factor. This may explain the reduced levels of IL-18 in gingival tissues from periodontitis patients as observed in our study.
A limitation of our study is that the levels of IL-17 and IL-18 in sites with moderate and severe attachment loss were not assessed and studies need to be done co-relating these cytokine levels with different degrees of chronic periodontitis severity.
CONCLUSION
It can be inferred from the study results, that IL-17 and IL-18 expression has considerable variation in periodontal health and disease. The exact role played by these cytokines in periodontal disease progression remains to be elucidated.
ACKNOWLEDGEMENT
The authors would like to thank the Central Research Facility of Sri Ramachandra University for providing the facilities for doing the research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–66. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 2.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin 18 and periodontal disease. J Dent Res. 2007;86:586–93. doi: 10.1177/154405910708600702. [DOI] [PubMed] [Google Scholar]
- 3.Belardelli F, Ferrantini M. Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol. 2002;23:201–8. doi: 10.1016/s1471-4906(02)02195-6. [DOI] [PubMed] [Google Scholar]
- 4.Gemmell E, Seymour GJ. Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000. 2004;35:21–41. doi: 10.1111/j.0906-6713.2004.003557.x. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 6.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–55. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 7.Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001;46:901–8. doi: 10.1016/s0003-9969(01)00057-7. [DOI] [PubMed] [Google Scholar]
- 8.Berglundh T, Liljenberg B, Lindhe J. Some cytokine profiles of T-helper cells in lesions of advanced periodontitis. J Clin Periodontol. 2002;29:705–9. doi: 10.1034/j.1600-051x.2002.290807.x. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Azuma T, Motohira H, Kinane DF, Kitetsu S. The potential role of interleukin-17 in the immunopathology of periodontal disease. J Clin Periodontol. 2005;32:369–74. doi: 10.1111/j.1600-051X.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 11.Vernal R, Dutzan N, Chaparro A, Puente J, Antonieta Valenzuela M, Gamonal J. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol. 2005;32:383–9. doi: 10.1111/j.1600-051X.2005.00684.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;41:541–9. doi: 10.1111/jcpe.12238. [DOI] [PubMed] [Google Scholar]
- 13.Biet F, Locht C, Kremer L. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med (Berl) 2002;80:147–62. doi: 10.1007/s00109-001-0307-1. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother. 2002;25(Suppl 1):S4–11. doi: 10.1097/00002371-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167:6568–75. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 17.Figueredo CM, Rescala B, Teles RP, Teles FP, Fischer RG, Haffajee AD, et al. Increased interleukin-18 in gingival crevicular fluid from periodontitis patients. Oral Microbiol Immunol. 2008;23:173–6. doi: 10.1111/j.1399-302X.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 18.Ozçaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46:592–8. doi: 10.1111/j.1600-0765.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 21.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Júnior WM, Rossi MA, et al. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 2009;24:1–6. doi: 10.1111/j.1399-302X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 23.Pradeep AR, Hadge P, Chowdhry S, Patel S, Happy D. Exploring the role of Th1 cytokines: Interleukin-17 and interleukin-18 in periodontal health and disease. J Oral Sci. 2009;51:261–6. doi: 10.2334/josnusd.51.261. [DOI] [PubMed] [Google Scholar]
- 24.Oda T, Yoshie H, Yamazaki K. Porphyromonas gingivalis antigen preferentially stimulates T cells to express IL-17 but not receptor activator of NF-kappaB ligand in vitro. Oral Microbiol Immunol. 2003;18:30–6. doi: 10.1034/j.1399-302x.2003.180105.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun. 2008;76:4206–13. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Hernández PE, Zamora-Perez AL, Fuentes-Lerma M, Robles-Gómez C, Mariaud-Schmidt RP, Guerrero-Velázquez C. IL-12 and IL-18 levels in serum and gingival tissue in aggressive and chronic periodontitis. Oral Dis. 2011;17:522–9. doi: 10.1111/j.1601-0825.2011.01798.x. [DOI] [PubMed] [Google Scholar]
- 27.Gemmell E, Carter CL, Hart DN, Drysdale KE, Seymour GJ. Antigen-presenting cells in human periodontal disease tissues. Oral Microbiol Immunol. 2002;17:388–93. doi: 10.1034/j.1399-302x.2002.170609.x. [DOI] [PubMed] [Google Scholar]
- 28.Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, et al. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest. 1998;101:595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001;166:4915–21. doi: 10.4049/jimmunol.166.8.4915. [DOI] [PubMed] [Google Scholar]
- 30.Udagawa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, et al. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–12. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]


