Abstract
With advanced technologies in hand, there exist potential applications and services built around monitoring activities of daily living (ADL) of elderly people at nursing homes. Most of the elderly people in these facilities are suffering from different chronic diseases such as dementia. Existing technologies are mainly focusing on non-medication interventions and monitoring of ADL for addressing loss of autonomy or well-being. Monitoring and managing ADL related to cognitive behaviors for non-medication intervention are very effective in improving dementia patients' conditions. However, cognitive functions of patients can be improved if appropriate recommendations of medications are delivered at a particular time. Previously we developed the Secured Wireless Sensor Network Integrated Cloud Computing for Ubiquitous-Life Care (SC3). SC3 services were limited to monitoring ADL of elderly people with Alzheimer's disease and providing non-medication recommendations to the patient. In this article, we propose a system called the Smart Clinical Decision Support System (CDSS) as an integral part of the SC3 platform. Using the Smart CDSS, patients are provided with access to medication recommendations of expert physicians. Physicians are provided with an interface to create clinical knowledge for medication recommendations and to observe the patient's condition. The clinical knowledge created by physicians as the knowledge base of the Smart CDSS produces recommendations to the caregiver for medications based on each patient's symptoms.
Key words: : medication recommendation, clinical decision support system, home health monitoring, dementia, telemedicine
Introduction
Various studies have reported that medical costs are growing rapidly and that a large proportion of this cost goes toward the elderly in society.1,2 Health information technologies are considered as one of the possible solutions to reduce these healthcare costs. Keeping in mind reducing the cost of healthcare, most nations, such as the U.S. Federal government, are investing about $27 billion in health information technologies.3 Furthermore, health information technology provides substantial savings by lowering cost if adverse effects of medications are managed properly.4
The most prominent costs associated with elderly people's health include those associated with falls and chronic diseases. Cognitive disorders are seen in most elderly people with increasing age.5 These cognitive disorders ultimately become severe and result in symptoms for dementia. The increase in the number of dementia patients becomes a social problem and at the same time increases healthcare costs.
In order to monitor activities of daily living (ADL) of the elderly in home healthcare, diverse sensor- and video-based monitoring technologies exist in the market. These monitoring technologies are playing a very positive role in the domain of telehealth and telecare for managing elderly people.6 Most of these technologies focus on monitoring patients' ADL, which assists caregivers or patients to manage non-medication activities and produces basic recommendations. Gokalp and Clarke7 identified that most of the existing monitoring systems address loss of autonomy or well-being such as meal preparation, personal hygiene, bathing, and dressing. These technologies are playing a pivotal role in improving elderly people's daily lives6; however, these services can improve patient care through integration with clinical recommendations and introducing physicians as observers of all these activities. As an example, Keranen and Liikkanen8 have developed a short message service–based medication reminder service for patients with Parkinson's disease. The service provides basic medication management and has positive effects on patients' health status, although it has not addressed the adherence to medications and involvement of physicians. Likewise, as indicated by other studies,9,10 incorporating a clinical decision support system (CDSS) for the dementia diagnosis can result in changing of work routines, increase teamwork, and enhance clinical knowledge of physicians.
In the Ubiquitous Computing Lab, Kyung Hee University, Yongin, Korea (http://uclab.khu.ac.kr/), we have developed a platform called Secured Wireless Sensor Network integrated Cloud Computing for ubiquitous-Life Care (SC3).11–13 SC3 supports different sensors that collect real-time data of a subject under observation and transmit to the cloud server through the cloud gateway. It has the capability to capture context information and derive high-level activities based on low-level sensory data.13 The system has been evaluated for monitoring Alzheimer's disease patients and provided with basic non-medication recommendations. It was lacking interfaces for medication intervention, and the physician role was ignored for managing patient activities.
In this article, we have proposed a service called Smart CDSS, which is integrated with the existing SC3 platform. Smart CDSS follows healthcare standards such as HL7 vMR and HL7 Arden Syntax, which helps in integration with diverse applications and sharing of clinical knowledge.14 The clinical knowledge base is the core component of the Smart CDSS service that allows physicians to express their experiences and publish already available clinical guidelines in a real domain environment using knowledge-authoring tools.15 Furthermore, the system is based on standard interfaces that allow diverse applications such as electronic medical records (EMRs)/electronic health records (EHRs) and other healthcare systems to interact for recommendations in the corresponding domain. For SC3 integration, the Adapter Human Activity Recognition Engine (HARE) was developed to take in patient activity information and produce appropriate recommendations. Moreover, it also supports Adapter Interoperability and Adapter EMR/EHR, which enable data and recommendation exchanges with clinical systems.16
The proposed system has a greater impact for the healthcare community. Patients are provided with healthcare services by processing sensor data collected related to them. Physicians are helped to create a knowledge base in standard format without knowing the technical details of the standards. Their involvement in monitoring patient health data improves patient care. Furthermore, incorporating clinical guidelines through decision support enhances skills for novice physicians in the corresponding clinical domain.
The cost of dementia patients' regular visits to the hospital due to minor complications can be resolved with the use of the proposed system, which will guide the caregiver in providing recommended care services without the patients' visits to the hospital. Furthermore, the existing system has the capability to consult physicians with high domain knowledge without visiting the patient's home health environment such as smart homes. As a result it reduces consultation costs of expert physicians.
In the rest of this article, we have elaborated the domain knowledge creation and transformation into shareable clinical knowledge. The example of dementia management has been derived from National Institute for Health and Clinical Excellence (NICE) guidelines17 and was provided with the derivation of the clinical workflow for dementia medication management of cognitive symptoms. Finally, the clinical workflow was transformed into shareable clinical knowledge using HL7 Arden Syntax.18
Dementia Management Guidelines and Interventions
Symptoms of Dementia Patients
Dementia is a broad term for a collection of symptoms that include loss of memory, mood change, and problems with communication and reasoning.19,20 It has many types; the most common are Alzheimer's disease and vascular dementia. It is diagnosed when two or more brain functions such as language and memory skills are impaired without loss of consciousness. During presentation of a dementia patient at clinical encounters, physicians strive to find out the following symptoms:
• Cognitive symptoms (for example, memory problem, communication problem, and disorientation)
• Psychiatric symptoms and personality change (for example, depression, anxiety, aggression, social withdrawal, restlessness, and abnormal beliefs)
• Neurological symptoms (for example, loss of ability to learn purposeful movements)
• Difficulties with ADL (for example, getting lost, forgetting recipes when cooking, loss of driving skills, and taking prescribed medications erratically)
Dementia Assessment and Diagnosis
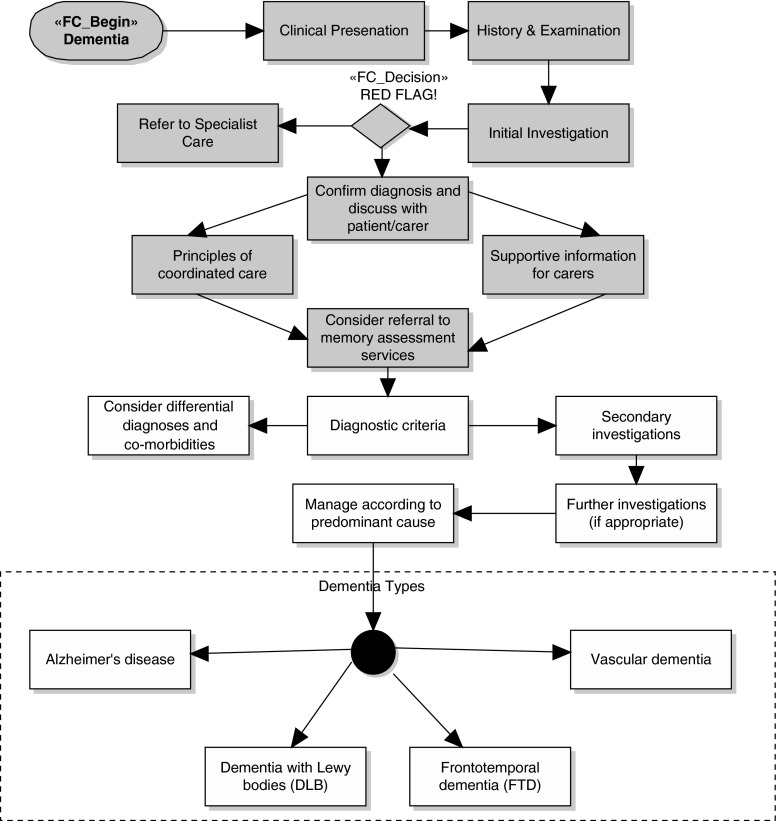
According to NICE guidelines17,21 for dementia, the assessment path can be shown as in Figure 1. In assessing the cause of dementia, the patient is passed through proper procedures. The patient is examined to insure the presence of dementia, by looking into his or her history about problems with memory, speech, communication, and alcohol consumption. Some basic pathology observations are also obtained like complete blood count, liver function tests, calcium, serum vitamin B12 level, and thyroid function tests. These investigations confirm the presence and severity of dementia, and patients are screened further to find the actual cause of dementia. In most cases, patients with a severe condition are referred to a specialist for intensive and immediate care. The most prominent causes of dementia include Alzheimer's disease, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia.
Fig. 1.
National Institute for Health and Clinical Excellence guidelines for dementia assessment.
Dementia Management
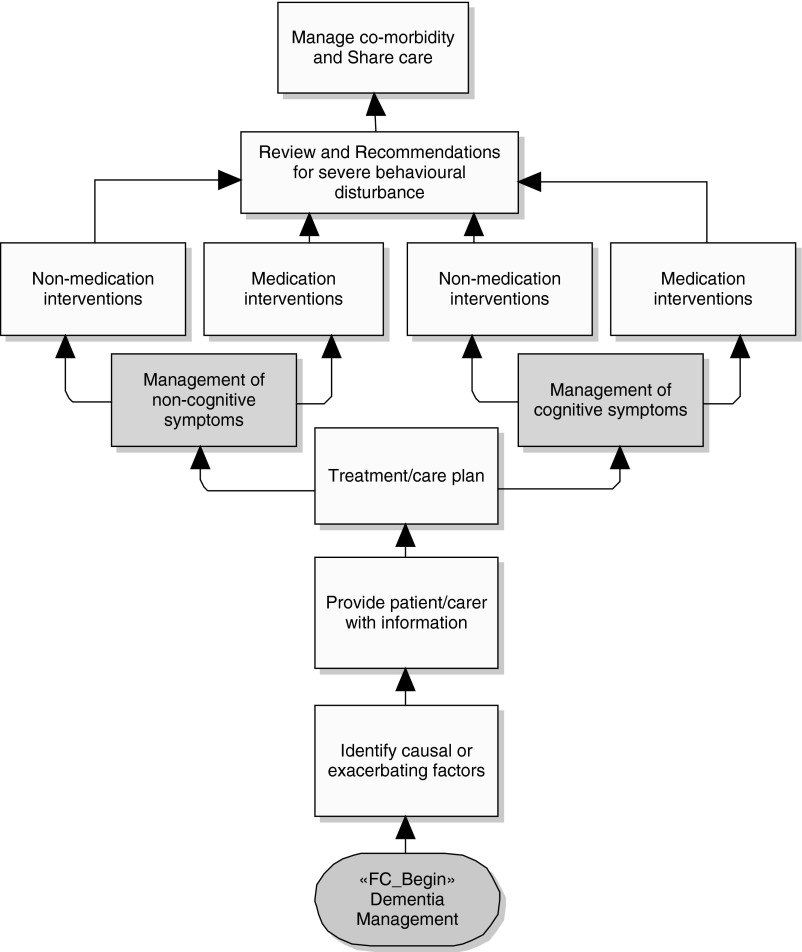
After assessment of the dementia, the specialist prepares a treatment and care plan for the patient in accordance with the cause and its severity. Figure 2 is the NICE guideline pathway partially depicting management of a dementia patient during treatment and care at a healthcare facility, nursing home, or home environment.
Fig. 2.
National Institute for Health and Clinical Excellence guidelines for dementia management.
In the treatment and care plan, the patient and caregivers are provided with guidelines to manage activities and treatment by observing closely the cognitive and noncognitive symptoms. Cognitive symptoms include problems with memory (short and long term), attention and concentration, eating and swallowing, language, and communication. Noncognitive symptoms include hallucinations, abnormal beliefs, extreme anxiety, and wandering.
Dementia Interventions
During the dementia management plan, the patient is observed for cognitive and noncognitive symptoms and severity. Interventions are planned for each category of symptoms as “Medication Intervention” and “Non-Medication Interventions.”
Cognitive symptoms: medication and non-medication interventions
Medication interventions are carefully planned with approval of an experienced specialist. The treatment is provided according to the cause of the dementia, and patients have the option of treatment with donepezil, galantamine, or revastigmine. Moreover, the caregiver should know the adverse effect of treatment, such as an Alzheimer's patient may face nausea and vomiting as the result of gastrointestinal effects due to treatment.
Non-medication interventions are offered for patients with mild-to-moderate dementia. Intentions of such interventions are targeted toward managing physiological needs such as managing pain, maintaining continence, and supporting sensory perception. Moreover, the patient is engaged in activities that simplify his or her daily life and provided with facilities that minimize communication problems, as well as environmental modifications are considered such as providing spaces for wandering and reducing stimulation.
Noncognitive symptoms: medication and non-medication interventions
Medication interventions for noncognitive symptoms are not recommended in normal conditions. According to NICE guidelines, the medications should be considered only in severe distress or if there is an immediate risk to the patient or others. However, the patient is closely monitored for any adverse effect of these medications. For example, using antipsychotic drugs may result in severe adverse reactions for patient with dementia with Lewy bodies and cause death for Alzheimer's disease, vascular, and mixed dementia patients.
A patient with dementia having noncognitive symptoms should have planned interventions that reflect his or her preferences, skills, and abilities. For distressing noncognitive symptoms, the patient and caregivers are provided with guidelines to take care of physical health, depression, side effects of medications, physical environmental factors, communication problems, and psychosocial factors. The intervention is planned to monitor the effect and response to the care plan and to adapt it in case of positive results. The care plan includes bright light therapy, multisensory stimulation, animal-assisted therapy, and therapeutic use of music/dancing and massage.
Knowledge Representation for Dementia Recommendations
Providing effective decision support services, knowledge engineering, and its representation become key factors to assure useful recommendations and alerts during interventions of patient care. In this view, the clinical decision support services should be regarded as a tool that helps the healthcare organization to deliver “right knowledge to the right people in the right form at right time.”22 This objective leads the CDSS developer to take into account (1) understanding organizational workflows where CDSS interventions are intended to be plugged in and (2) appropriate knowledge modeling and representation so that the CDSS can deliver suitable recommendations for patient care.
In our research work, we are providing medical interventions in a home health sensor-based environment and direct recommendations to patient caregivers (physicians or nurses) through standard HL7 interfaces of the EMR. For non-medical interventions, the normal recommendations are displayed on a screen, or some actions are taken according to situations observed in the patient care environment. For knowledge representations, we are incorporating HL7 Arden Syntax with the intention to provide a clinical knowledge base that
• Can be shareable across organization boundaries
• Allows meta information to maintain the knowledge base and link it to literature
• Facilitates mapping of vocabulary terms in the local domain to terms in the knowledge base
Overview of HL7 Arden Syntax
HL7 Arden Syntax is a widely used and recognized standard for representing clinical and scientific knowledge that can be understandable by physicians and executed for alerts and recommendations in clinical decision support. The HL7 Arden Syntax baseline was established in 1992 by the American Society for Testing and Materials and was adapted later in 1999 by HL7. Recently HL7 has published HL7 Arden Syntax version 2.8 for future enhancements.18
Arden Syntax provides a standard base format that resembles natural language without dependency on a common programing language and any particular implementation of a specific healthcare system. This key feature turns to make it easier in understanding for nonexperts. Moreover, the nondependency on healthcare systems makes it feasible to exchange clinical decision support knowledge across different systems and sites.18
Arden Syntax focuses on representing knowledge bases in sets of Medical Logic Modules (MLMs). It helps in representing independent rules, formulas, or protocols in an amenable set of MLMs. The MLM encapsulates knowledge as software modules that trigger an action based on a data event generated at the healthcare system.23 Initially the MLM was intended to have single logic that acts on a single set of data and results in a single set of actions. However, now it can support other MLMs that result in chaining of actions with their own logic and set of data elements.
HL7 Arden Syntax specification defines a standard format for MLM. Each MLM contains slots that are logically grouped into three required categories and one optional category23,24:
• maintenance
• library
• knowledge
• resources (optional)
MLM “maintenance” contains subslots used for maintenance and change control. These slots contain information not related to knowledge in the MLM. It includes slots such as title (MLM title), author (MLM writer), and specialist (person responsible for validating MLM).
The MLM “library” contains subslots used for knowledge base maintenance that are related to the MLM's knowledge. It helps in searching through a knowledge base of MLMs. Example slots includes purpose (define the purpose of the MLM), key words (key words helping in searching the MLM), and citations (link to resources of knowledge), among others.
MLM “knowledge” contains slots that specify the intention of what the MLM does. Its subslots include a data slot (define terms used in MLM), evoke slot (specify context of MLM evocation), logic slot (the actual condition to be tested on terms), and action slot (specify the action that should be taken in case the condition is true).
MLM “resource” is an optional category that contains a set of language slots to localize messages of recommendations in different languages. Example slots include default (default language code) and language (user-preferred language code).
Representation of Guidelines for Medication Intervention in Cognitive Symptoms
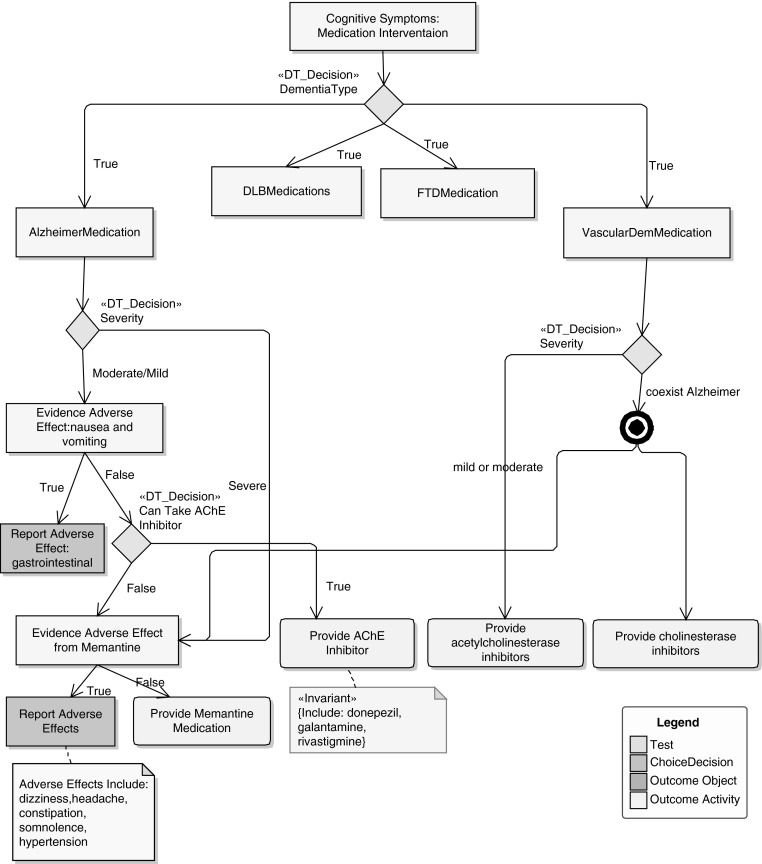
Considering medication intervention for cognitive symptoms, Figure 3 depicts the recommendation of different medication plans for a patient with dementia caused by Alzheimer's disease and vascular problems. Based on patient symptoms, allergies, and other conditions, the decision tree (derived from NICE guidelines) provides an appropriate medication plan for the patient. As an example, patients having dementia caused by Alzheimer's disease will be given an acetylcholinesterase (AChE) inhibitor provided that they have moderate or mild symptoms, no evidence of adverse effects of nausea and vomiting, and the capability to absorb the AChE inhibitor.
Fig. 3.
Medication intervention for cognitive symptoms for Alzheimer's disease and vascular dementia. AChE, acetylcholinesterase; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia.
In order to represent these guidelines in standard format, one way to build a shareable knowledge base is by creating five MLMs:
• One MLM “DemMedInt_root” representing the main root logic of medication interventions. The “DemMEdInt_root” MLM is evoked when the dementia patient is given medications based on his or her status. It parses the input to find the dementia symptoms, and based on the type of dementia, it calls the appropriate sub-MLM (for the detailed logic of the MLM, see the Appendix).
• Four MLMs for individual medication plans for each type of dementia. Two of the MLMs are “mlmAlzDementia” and “mlmVascDementia,” reflecting logic from Figure 3 representing Alzheimer's disease and vascular dementia medications, respectively (for the detailed logic of the MLM, see the Appendix).
• The root MLM will cause the triggering of the corresponding MLM matching the type of dementia.
Existing SC3 Environment
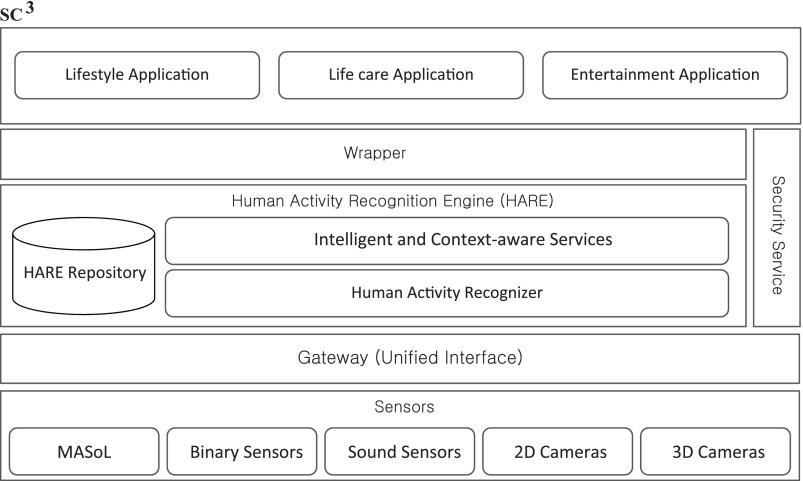
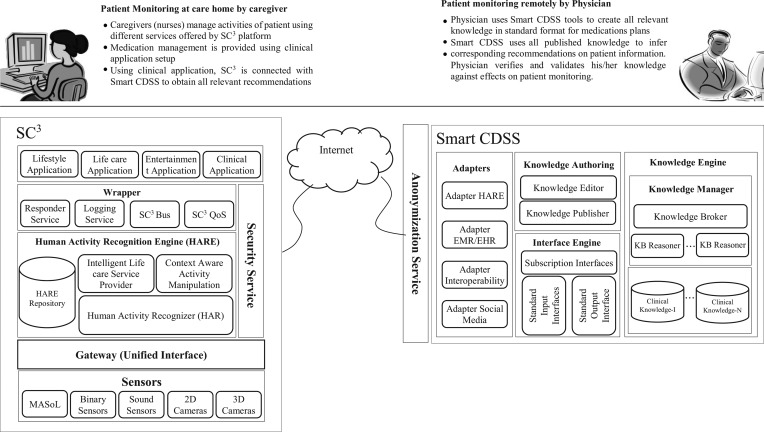
SC3 is a cloud-based platform architecture designed and developed in the Kyung Hee University Ubiquitous Computing Lab, with the main objective of providing recommendations based on activities recognized for Alzheimer's disease patients.11–13 The SC3 framework is divided into different layers from life care-related applications to embedded devices as shown in Figure 4:
Fig. 4.
Secured Wireless Sensor Network Integrated Cloud-Computing for Ubiquitous-Life Care (SC3). 2D, two-dimensional; 3D, three-dimensional.
• The sensor layer, at the very bottom, is used to collect information about the user's activities. These include binary and sound sensors and two- and three-dimensional cameras. These sensory and camera devices recognize low-level activities that are used for inferring high-level activities by the HARE.
• Above the sensor layer is the data processing layer, which provides primitive recommendations. These recommendations would be of great help to the patients, but some of them might contradict clinical information of the patient, thus limiting the scope of the system deployed. The HARE Repository stores the activities recognized by the sensor layers. The Human Activity Recognizer recognizes these activities and provides low-level sensory data information to the Context Aware Manipulation Engine module. HARE uses different approaches for activity recognition to find activities such as an embedded sensor-based activity recognizer, a wearable sensor-based activity recognizer, a two-dimensional camera-based activity recognizer, and a three-dimensional camera-based activity recognizer. These sensor and camera devices recognize low-level activities that are used for inferring high-level activities by Context Aware Manipulation Engine, for providing recommendations.
• The wrapper layer provides security services to use the system by maintaining quality of services and privacy issues. These include responder services, logging services, and SC3 Bus services and SC3 quality of services.
• Users can communicate with the SC3 system by utilizing the wrapper layer. This layer includes applications such as Lifestyle, Life Care, and Entertainment applications.
SC3 provides recommendations based on activities of a patient, but these are very basic-level activities. Recommendations in the current SC3 are not taking clinical information into account and, therefore, may conflict with the condition of the patient. To facilitate clinical information usage with activities recognized, integration with current EMR systems is necessary. Integration requires conversion to and from the healthcare standard format, which is another challenge for extending the SC3 system.
Proposed System
Extended SC3 Environment
We propose an approach for extending our SC3 platform to combine the patient's activities information with clinical information for better decision-making. We investigated and identified different aspects for extension of the current SC3 setup and redesigned the system as shown in Figure 5. Initially, the format of the HARE Repository was redesigned to effectively store the activities recognized for monitoring the patient. Second, the clinical data of the same patient were imported from an EMR compliant to a particular standard (in this case we assume the EMR is compliant with HL7 CDA). Third, the activities and clinical information were combined in a standard format (called HL7 vMR) for decision support. Lastly, guidelines were generated in Arden Syntax format, and recommendations were provided to patients in an easy-to-understand format.
Fig. 5.
Smart Clinical Decision Support System (CDSS): extended architecture view of Secured Wireless Sensor Network Integrated Cloud-Computing for Ubiquitous-Life Care (SC3). 2D, two-dimensional; 3D, three-dimensional; EHR, electronic health record; EMR, electronic medical record; QoS, quality of services.
Recommendation Service Model: Smart CDSS
Smart CDSS is a clinical recommendation service that provides recommendations based on the patient's symptoms and clinical information using clinical knowledge maintained by physicians. Smart CDSS adapts existing interoperability standards to allow interactions with diverse types of consumer applications. It comes with an Adapters module to transform incoming patient information into the Smart CDSS–understandable format. Smart CDSS follows HL7 vMR as the reference standard for the internal data model used by clinical knowledge. The SC3 framework is supported through Adapter HARE, which transforms the patient's activities data into HL7 input vMR format and vMR-based recommendations into SC3 format. Moreover, Smart CDSS also supports recommendations for HL7-based healthcare systems such as the EMR and the EHR. Figure 5 shows the modular view of Smart CDSS.
The Knowledge Engine module includes clinical knowledge repositories and a corresponding knowledge reasoner. Clinical knowledge comprises compiled Arden Syntax–based MLMs published by physicians. These compiled MLMs have detailed metadata information recorded by the Knowledge Manger. KB Reasoners are invoked for incoming patient information, received as a result of some event, and ultimately schedule all related MLMs for execution. Recommendations generated via executed MLMs are transformed to a consumer application via the standard output interface and the specified adapter. As an example, in the SC3 environment patient status information is received via Adapter HARE and Adapter EMR/EHR through the caregiver's request. This information is transformed into HL7 vMR format and forwarded to the Knowledge Engine through the standard input interface. The Knowledge Engine allows KB Reasoners to schedule appropriate dementia medication MLMs and produce recommendations. As a result of matched MLMs, the prescribed medication recommendations are returned as vMR output to Adapter HARE. The Adapter HARE returns these recommendations in an SC3-compliant format to the caregiver.
Smart CDSS is equipped with authoring tools that allow physicians to create and publish clinical knowledge.15 The authoring tool is provided with knowledge editor and compilation modules that enable the physician to create Arden-based shareable clinical knowledge as a set of MLMs.
Integration with EMR System
Clinical information about dementia patients in the EMR compliant with HL7 CDA requires integration for effectively utilization of the activities recognized. The patient's data are obtained from the EMR system in HL7 CDA format and are converted to HL7 vMR format for integration with our proposed system.16 The patient can perform activities that require a response by decision support based on medication. This decision requires physician consultation; therefore the medication that the decision support system finds out is communicated with the EMR system in the form of an alert to the physician. The physician evaluates the recommendation and approves the appropriate medication that is recommended to the patient by the proposed system.
Integration requires the EMR system's data format conversion into the proposed system's format (i.e., vMR format). The proposed system can only process information in vMR format for generating recommendations. On the other hand, conversion is also necessary from vMR format to EMR system format when communication is necessary with the EMR system. The EMR system format is dependent on standard compliancy or legacy data formats. It can be compliant with the HL7 CDA standard, thus requiring a conversion mechanism from HL7 CDA to vMR, and vice versa.
System Realization: Case Study
Workflow of the management of dementia patients covers management of cognitive symptoms that consists of two sections: Non-Medication Interventions and Medication Interventions, as shown in Figure 2. Decision support for both these subsections is necessary based on the severity of the patient's condition. In order to cover both medication and non-medication interventions, we have set up the system in the following manner:
1. Sensors and cameras-based monitoring environment. Management of a dementia patient's lifestyle is handled by monitoring his or her activities using sensors and cameras. In order to make use of such a monitoring environment, we have used the already existing SC3 infrastructure as explained in the section Existing SC3 Environment. Non-medication interventions require monitoring the activities of a dementia patient that can result in catastrophic outcomes. One of the symptoms of a dementia patient is forgetting things quickly, such as during cooking he or she turns on the stove and leaves it without turning it off. To manage such a situation, we embed the stove with a motion sensor that takes notice of turning the stove on and off. The decision support calculates the estimated time of cooking, and after that if the stove is not turned off, it makes the decision to turn it off. There are other examples as well for non-medication interventions for managing a dementia patient's activities.
2. Patient medical record management for medication intervention. Another scenario is for medication interventions that find the severity of a patient to be manageable only using medication. As it is directly related to the patient's health and can have an adverse effect, therefore decision support cannot directly suggest any recommendation to the patient. To provide proper medication interventions, it is important to know the patient's health history and current medication plan from his or her medical record. To keep the system interoperable with most of the existing EMR, we have developed an interoperable framework to work with different HL7 standards such as HL7 CDA. It consumes the patient medical record in the HL7 CDA format and has the capability to parse the patient history and other related information of medications.
3. Recommendation service for medication intervention. Medication intervention needs proper engagement of physicians and requires proper monitoring of the patient after prescribed medications are dispensed. We have provided a physician panel in the proposed system that can regularly observe the patient's conditions for prescribed medications. Furthermore, we have also facilitated physicians to provide already proved clinical practices as clinical knowledge that can be activated based on the patient's symptoms to provide various medication interventions. This capability in the existing SC3 environment has been extended with the recommendation service called Smart CDSS. Physicians are provided with tools to create clinical knowledge that becomes part of the Smart CDSS knowledge base, and it can also be shared across the organization. We have trained the physicians through transforming their domain knowledge into an executable and shareable format using HL7 Arden Syntax. The sections Dementia Management Guidelines and Interventions and Knowledge Representation for Dementia Recommendations mainly explain the methodology for knowledge transformation.
To realize the system as a whole for medication and non-medication interventions, we have depicted the business process flow among various modules of the system in Figure 6. It depicts all stakeholders as participants who perform various activities to provide the monitoring environment with approved recommendations under the close observation of physicians.
Fig. 6.
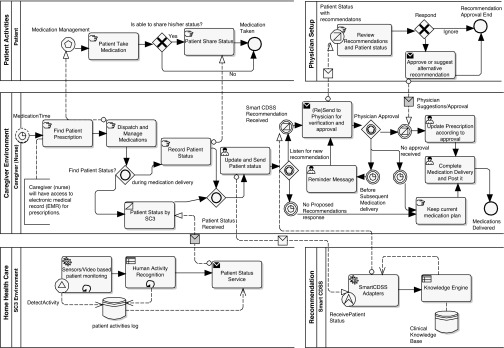
Patient medication monitoring workflow. Patient medication monitoring workflow is represented in the Business Process Model and Notation (www.bpmn.org) process model where the set of activities is represented as a pool using Enterprise Architect (www.sparxsystems.com/products/ea/). CDSS, Clinical Decision Support System; SC3, Secured Wireless Sensor Network Integrated Cloud-Computing for Ubiquitous-Life Care.
Patient Activity Pool
The patient is the main subject of the system; the extended system provides the services of monitoring the patient's activities and produces useful recommendations for managing medications and non-medication activities. For the medication scenario, two activities of patients are important for safe recommendations. It includes “Patient Take Medication” and “Patient Share Status.” “Patient Take Medication” covers proper delivery of medications at various medication times initiated by the patient or the caregiver. “Patient Share Status” involves the patient's status narrated by the patient to the caregiver. This activity includes any medication effect on the patient during the last dose interval and is considered only if the patient is able to share his or her status.
Physician Setup Pool
The physician plays a pivotal role in managing medications and other aspects of the patient's status remotely and is a key participant in the home healthcare environment. The physician is performing a dual role in the process. First, the physician maintains the Smart CDSS knowledge base with up-to-date guidelines that trigger recommendations for the patient under observation in the home healthcare environment. Second, he or she validates and verifies the Smart CDSS recommendations generated based on the patient status obtained via SC3 monitoring services and the updated status from the caregiver. In the current scenario, the pool represents only activities for verification and approval of recommendations. Details of dementia knowledge representation and transforming it into equivalent HL7 Arden Syntax are discussed in Representation for Dementia Recommendations.
The physician performs the “Review Recommendations and Patient Status” activity to verify and approve recommendations of the Smart CDSS submitted by the caregiver. The physician responds to the caregiver with approval or suggestions if needed via the “Approve or Suggest Alternative Recommendation” activity.
Caregiver Environment Pool
Caregivers (nurses) are the main participants in coordinating patient activities, patient status, managing medications, and candidate recommendations with the physician. During the phase of medication management and finding appropriate recommendations, the caregiver performs the following activities in sequence:
• The caregiver receives the prescription for the patient from the EMR. This activity is indicated as “Find Patient Prescription.” We are providing an interface in the system to integrate the Smart CDSS to import data from any HL7 CDA–compliant EMR.
• According to the prescription for a particular patient, the caregiver dispatches the medication and deliver it to the patient at his or her site. The “Dispatch and Manage Medications” activity represents the caregiver's actions in the delivery process.
• After the caregiver dispatches and delivers the medications, his or her next activity is to record the patient status that indicates effects of the medications from previous dosages. The patient status can be obtained by the caregiver directly from the patient at bedside, or the patient's noncognitive behaviors can be obtained from the SC3 environment. These activities are modeled as “Record Patient Status” and “Patient Status by SC3,” respectively. Furthermore, the inclusive gateway “Find Patient Status?” provides representation that the caregiver expects patient status information from one resource at least and will proceed to the next activities after receiving the status represented by the “Patient Status Received” inbound inclusive gateway.
• The caregiver updates the patient status provided by the patient at the site and/or obtained from the patient-monitoring SC3 environment. In order to obtain further recommendations based on the patient's current status, the caregiver submits the patient status to the Smart CDSS service. The “Update and Send Patient Status” activity represents these tasks of the caregiver.
• The caregiver waits for possible recommendations from the Smart CDSS service. It has been modeled as an event-based gateway, “Listen for New Recommendation,” where it triggers the next activity depending on the response from the Smart CDSS recommendation service.
• The caregiver completes and posts medication delivery and comes up with the current prescription plan for next time of medications delivery in case no proposed recommendations are received from the Smart CDSS service. These activities are represented as “Keep Current Medication Plan” and “Complete Medication Delivery and Post It.”
- • The caregiver submits recommendations (if any) received from the Smart CDSS service to the physician for approval or alternate suggestions. This message is conveyed to the physician via the integrated system, and the caregiver waits for any expected approval. This activity is modeled as “Send to Physician for Verification and Approval,” and the event-based gateway “Physician Approval” is used to receive the approval. The caregiver will expect any approval or suggestions from the physician and will wait for it for some time. During this time interval, he or she may perform any of the following set of activities:
- ○ The physician responds with approval or alternate suggestions as indicated by the message received with the notation “Physician Suggestions/Approval.” The caregiver updates the existing medication plan according approval or suggestions, finishes the medication delivery, and posts it. These activities are represented with “Update Prescription According to Approval” and “Complete Medication Delivery and Post It.”
- ○ To ensure that the physician has seen the Smart CDSS recommendations, the caregiver resends the recommendations as a reminder after some time interval. “Reminder Message” and “Resend to Physician for Verification and Approval” activities are performed by the caregiver to ensure that somehow the physician has not missed viewing the previous recommendation message.
- ○ If physician approval is not received after the reminder message, then the caregiver expects that the recommendation was not somehow important with respect to the patient's condition and has been ignored by the physician. He or she keeps the current medication plan and completes the medication delivery.
Home Healthcare Pool
The SC3 environment is the main infrastructure equipped with sensory devices developed at Ubiquitous Computing Lab to monitor patients with home healthcare for their activities. The main features of this system are to observe and monitor noncognitive activities of the patient using various sensors and video support. SC3 monitors patient activities on a regular basis and maintains the activities log, where a high-level context aware system enables deriving high-level activities of the patient. These high-level activities are represented as noncognitive behavior of the patient and are shared with the caregiver during care of the patient.
The whole process of monitoring is represented as the loop activities of “Sensor/Video Based Patient Monitoring” and “Human Activity Recognition.” The high-level context of patient activities is shared as the patient's status message with the caregiver using the “Patient Status Service” activity.
Recommendation Pool
Smart CDSS is extended service in the SC3 environment that facilitates integration of the recommendations service for monitoring cognitive behavior of the patient in home healthcare. Furthermore, it also allows physicians to keep in touch with home healthcare in an integrated environment. Smart CDSS services are provided with clinical knowledge established from the expertise of the physician and are derived from published resources as guidelines in the dementia domain. It produces recommendations after receiving the patient's symptoms and status based on the available knowledge base. The main processes involved in recommendation are “Smart CDSS Adapters” and “Knowledge Engine,” which provide integration with external patient monitoring systems and generate recommendations, respectively.
Conclusions and Future Work
Clinical recommendations and involvement of the physician in monitoring systems for the elderly improve patient safety. Providing timely treatment and medication management can avoid having the patient suffer from other comorbidities. The proposed system supports a recommendation service based on the physician's clinical knowledge and also have support for integration with other healthcare systems for investigating the medical record of the patient. This extension of Smart CDSS enhances the capability of the SC3 platform to monitor and manage ADL of elderly people with dementia under close observation by a physician. As a result of its extension for medical intervention, the system improves overall management of dementia patients. Moreover, the system can also reduce healthcare costs by avoiding clinic visits by patients. Associating with expert physicians remotely allows timely delivery of patient care, which enhances patient safety and saves costs incurred due to physician visits.
As with continuous monitoring data from a sensor environment and integration with the patient medical record for medication interventions, the data volume is becoming huge. The existing infrastructure has limitations in managing a huge volume of data. We are planning to extend the existing framework to support big data where we can enable various health analytics on patient data in real time and offline.
Appendix
MLM-1: ROOT MLM “DEMMEDINT_ROOT” FOR DEMENTIA MEDICAL INTERVENTION
Explanation
This MLM intends to consume a dementia patient's clinical information including problems, allergies, and different observation results and medications history. Depending on the patient's conditions and symptoms, it categorizes the patient into a particular type of dementia and transfers patient data to corresponding sub-MLMs for further medications management.
maintenance:
title: Dementia Medical Intervention MLM;;
mlmname: DemMedInt_root;;
arden: ASTM-E1460-1995;;
version: 2.7;;
institution: UC Lab;;
author: Maqbool Hussain;;
specialist: ;;
date: 2012-12-02;;
validation: Prototype Testing;;
library:
purpose: This MLM is triggered for medical intervention for the dementia patient;;
explanation: This MLM is evoked when some activities of dementia patients are observed during monitoring in the home-health environment for medication;;
keywords: dementia; medications; treatment plan;;
citations: NICE Guidelines;;
knowledge:
type: data-driven;;
data:
/* Observation from monitoring the dementia patient in the home-health environment evokes this MLM */
medication_plan :=event {medication_order where class=dementia};
PatientClinicalStatement :=object [Problem, AdverseEvent, Observations, SubstanceAdministration];
patientCurrentStatus :=read as PatientClinicalStatement
{ select problem, adverseEffects, observationResults from client };
mlmAlzheimer :=MLM 'mlmAlzDementia.mlm';
mlmVascular :=MLM 'mlmVascDementia.mlm';
mlmDLB :=MLM 'mlmDLBDementia.mlm';
mlmFTD :=MLM 'mlmFTDDementia.mlm';
;;
evoke:
medication_plan;;
logic:
Problem=patientCurrentStatus.Problem;
/*Calling Alzheimer MLM when Alzheimer is observed…*/
IF Problem.problemCode imer” Then
outPutClinicalStatement :=call mlmAlzheimer with patientCurrentStatus;
ElseIF Problem.problemCode=“Vascular” Then
/*Calling Vascular Dementia MLM when Vascular is observed…*/
outPutClinicalStatement:=call mlmVascular with patientCurrentStatus;
ElseIF Problem.problemCode=“DLB” Then /*Calling DLB Dementia MLM when DLB is observed…*/
outPutClinicalStatement :=call mlmDLB with patientCurrentStatus;
ElseIF Problem.problemCode=“FTD” Then /*Calling FTD Dementia MLM when FTD is observed…*/
outPutClinicalStatement :=call mlmFTD with patientCurrentStatus;
EndIF;
IF outPutClinicalStatement IS PRESENT Then
CONCLUDE true;
EndIF;
;;
action:
IF outPutClinicalStatement.SubstanceAdministration IS PRESENT Then
Write “Patient Has Problem “|| Problem || “. Medications Suggested:“
|| outPutClinicalStatement.SubstanceAdministration.subtance;
Else
Write “Patient Has Problem “|| Problem || “.Patient should be monitored for Adverse Effects:“
|| outPutClinicalStatement.AdverseEvent;
EndIF;
;;
End;
MLM-2: ALZHEIMER MEDICATION MLM “MLMALZDEMENTIA”
Explanation
This MLM supports the medication plan for a dementia patient with Alzheimer's disease symptoms. It intends to consume patient allergies (if any) and already administered medications (if any). Based on the allergies record and previous medication dispensed, it suggests further the most appropriate medication plan.
maintenance:
title: Alzheimer's Medication Intervention MLM;;
mlmname: mlmAlzDementia;;
arden: ASTM-E1460-1995;;
version: 2.7;;
institution: UC Lab;;
author: Maqbool Hussain;;
specialist: ;;
date: 2012-12-06;;
validation: Prototype Testing;;
library:
purpose: This MLM is triggered for medical intervention for Alzheimer medication;;
explanation: This MLM is evoked to suggest medications and report adverse effects of medication for an Alzheimer's disease patient monitored in the home health environment. It suggests memantine or AChE inhibitor based on Alzheimer's disease severity and other adverse effects;;
keywords: dementia; medications; treatment plan, Alzheimer;;
citations: NICE Guidelines;;
knowledge:
type: data-driven;;
data:
RecommendationClinicalStatement :=object [AdverseEvent, SubstanceAdministration];
recClinicalStatement :=NEW RecommendationClinicalStatement;
/*MLM expecting patient clinical statement as parameter*/
patientClinicalStatement :=ARGUMENT;
;;
evoke:
/* This MLM will be called by Root Dementia MLM: ‘DemMedInt_root,’ which triggers the medication plan during treatment of a dementia patient */;;
logic:
IF patientClinicalStatement.Problem.Severity=“Severe” Then
IF patientClinicalStatement.AdverseEvent.adverseEventCode IS IN (“dizziness”, “headache”,
“constipation”, “somnolence”, “hypertension” ) AND
patientClinicalStatement.AdverseEvent.adverseEventAgent=“Memantine” Then
recClinicalStatement.AdvereEvent=patientClinicalStatement.AdverseEvent;
CONCLUDE true;
Else
recClinicalStatement.SubstanceAdministration.subtance=“Memantine”;
CONCLUDE true;
EndIF;
ElseIF patientClinicalStatement.AdverseEvent.adverseEventCode IS IN(“nausea”, “vomiting”) Then
recClinicalStatement.AdvereEvent=patientClinicalStatement.AdverseEvent;
CONCLUDE true;
ElseIF patientClinicalStatement.AdverseEvent.adverseEventStatus=“Active” AND
patientClinicalStatement.AdverseEvent.adverseEventAgent=“ACHE Inhabitor” Then
IF patientClinicalStatement.AdverseEvent.adverseEventCode IS IN (“dizziness”, “headache”,
“constipation”, “somnolence”, “hypertension” ) AND
patientClinicalStatement.AdverseEvent.adverseEventAgent=“Memantine” Then
recClinicalStatement.AdvereEvent=patientClinicalStatement.AdverseEvent;
CONCLUDE true;
Else
recClinicalStatement.SubstanceAdministration.subtance=“Memantine”;
CONCLUDE true;
EndIF;
Else
recClinicalStatement.SubstanceAdministration.subtance=“ACHE Inhibitor”;
CONCLUDE true;
EndIF;
;;
action:
RETURN recClinicalStatement;
;;
End;
MLM-3: VASCULAR MEDICATION MLM “MLMVASCDEMENTIA”
Explanation
This MLM supports the medication plan for a dementia patient with vascular symptoms. It intends to consume patient allergies (if any) and already administered medications (if any). Based on the allergies record and previous medication dispensed, it suggests further the most appropriate medication plan.
maintenance:
title: Vascular Dementia Medication Intervention MLM;;
mlmname: mlmVascDementia;;
arden: ASTM-E1460-1995;;
version: 2.7;;
institution: UC Lab;;
author: Maqbool Hussain;;
specialist: ;;
date: 2012-12-08;;
validation: Prototype Testing;;
library:
purpose: This MLM is triggered for medical intervention for vascular dementia medication;;
explanation: This MLM is evoked to suggest medications and report adverse effects of medication for the vascular dementia patient monitored in the home-health environment. It suggests memantine, AChE inhibitor, or cholinesterase inhibitor based on vascular dementia severity. Based on patient current status it will alert for all adverse effects;;
keywords: dementia; medications; treatment plan, vascular dementia;;
citations: NICE Guidelines;;
knowledge:
type: data-driven;;
data:
RecommendationClinicalStatement :=object [AdverseEvent, SubstanceAdministration];
recClinicalStatement :=NEW RecommendationClinicalStatement;
/*MLM expecting patient clinical statement as parameter*/
patientClinicalStatement :=ARGUMENT;
;;
evoke:
/* This MLM will be called by Root Dementia MLM: ‘DemMedInt_root,’ which triggers the medication plan during treatment of a dementia patient */;;
logic:
IF patientClinicalStatement.Problem.Severity=“Severe” Then
IF patientClinicalStatement.Problem.relatedClinicalStatement.problemCode=“Alzheimer”
Then
recClinicalStatement.SubstanceAdministration[0].subtance=“cholinesterase inhibitor”;
IF patientClinicalStatement.AdverseEvent.adverseEventCode IS IN (“dizziness,” “headache,” “constipation,” “somnolence,” “hypertension”) AND patientClinicalStatement.AdverseEvent.adverseEventAgent=“Memantine” Then
recClinicalStatement.AdvereEvent=patientClinicalStatement.AdverseEvent;
Else
recClinicalStatement.SubstanceAdministration[1].subtance=“Memantine”;
EndIF;
CONCLUDE true;
EndIF;
Else
recClinicalStatement.SubstanceAdministration.subtance= “acetylcholinesterase inhibitors”;
CONCLUDE true;
EndIF;
;;
action:
RETURN recClinicalStatement;
;;
End;
Acknowledgments
This research was supported by the Ministry of Science, ICT & Future Planning of Korea under the Information Technology Research Center support program supervised by the National IT Industry Promotion Agency (NIPA) [grant NIPA-2013-(H0301-13-2001)].
Disclosure Statement
No competing financial interests exist.
References
- 1.Lehnert T, et al. Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev 2011;68:387–420 [DOI] [PubMed] [Google Scholar]
- 2.Fuchs VR. Health care for the elderly: How much? Who will pay for it? Health Aff (Millwood) 1999;18:11–21 [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–504 [DOI] [PubMed] [Google Scholar]
- 4.Evidence on the costs and benefits of health information technology. Publication No. 2976. 2008. Available at www.cbo.gov/sites/default/files/cbofiles/ftpdocs/91xx/doc9168/05-20-healthit.pdf (last accessed July26, 2013)
- 5.Suzuki T, Murase S. Influence of outdoor activity and indoor activity on cognition decline: Use of an infrared sensor to measure activity. Telemed J E Health 2010;16:686–690 [DOI] [PubMed] [Google Scholar]
- 6.Pecina JL, et al. Telemonitoring increases patient awareness of health and prompts health-related action: Initial evaluation of the Tele-ERA study. Telemed J E Health 2011;17:461–466 [DOI] [PubMed] [Google Scholar]
- 7.Gokalp H, Clarke M. Monitoring activities of daily living of the elderly and the potential for its use in telecare and telehealth: A review. Telemed J E Health 2013;19:910–923 [DOI] [PubMed] [Google Scholar]
- 8.Karenen T, Liikkanen S. Medication reminder service for mobile phones: An open feasibility study in patients with Parkinson's disease. Telemed J E Health 2013;19:888–890 [DOI] [PubMed] [Google Scholar]
- 9.Lindgren H. Towards personalized decision support in the dementia domain based on clinical practice guidelines. User Modeling User Adapted Interact 2011;21:377–406 [Google Scholar]
- 10.Lindgren H. Limitations in physicians' knowledge when assessing dementia diseases—An evaluation study of a decision-support system. Stud Health Technol Inform 2011;169:120–124 [PubMed] [Google Scholar]
- 11.Le XH, et al. Secured WSN-integrated cloud computing for u-life care. CCNC 2010, 7th IEEE Consumer Communications and Networking Conference (CCNC). New York: IEEE, 2010:1–2 [Google Scholar]
- 12.Khattak AM, et al. Context-aware human activity recognition and decision making. HealthCom 2010. IEEE 12th International Conference on e-Health Networking, Application Services. New York: IEEE, 2010:112–118 [Google Scholar]
- 13.Khattak AM, et al. Towards smart homes using low level sensory data. J Sensors 2011;11:11581–11604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain M, Khan WA, Afzal M, Lee S. Smart CDSS for smart homes. In: Donnelly M, Paggetti C, Nugent C, Mokhtari M, eds. Impact analysis of solutions for chronic disease prevention and management. Lecture Notes in Computer Science, Volume 7251. New York: Springer, 2012:266–269 [Google Scholar]
- 15.Ali T, Hussain M, Khan WA, Afzal M, Lee S. Authoring tool: Acquiring sharable knowledge for smart CDSS. EMBS 2013. IEEE 35th Annual International Conference of the EMBS. New York: IEEE, 2013:1278–1281 [DOI] [PubMed] [Google Scholar]
- 16.Khan WA, Hussain M, Amin B, Khattak AM, Afzal M, Lee S. AdapteR Interoperability ENgine (ARIEN): An approach of interoperable CDSS for ubiquitous healthcare. In: Urzaiz G, Ochoa G, Bravo J, Chen LL, Oliveira J, eds. Ubiquitous computing and ambient intelligence. Context-awareness and context-driven interaction. Lecture Notes in Computer Science, Volume 8276. New York: Springer, 2013:247–253 [Google Scholar]
- 17.NICE pathways: Dementia overview. Available at http://pathways.nice.org.uk/pathways/dementia (last accessed June10, 2013)
- 18.Samwald M, Fehre K, De Bruin J, Adlassnig KP. The Arden Syntax standard for clinical decision support: Experiences and directions. J Biomed Inform 2012;45:711–718 [DOI] [PubMed] [Google Scholar]
- 19.About dementia. Available at www.alzheimers.org.uk/site/scripts/documents.php?categoryID=200120 (last accessed August25, 2013)
- 20.NINDS dementia information page. Available at www.ninds.nih.gov/disorders/dementias/dementia.htm (last accessed August25, 2013)
- 21.Dementia assessment. Available at http://healthguides.mapofmedicine.com/choices/map-open/dementia1.html (last accessed May29, 2012)
- 22.Peleg M, Tu S. Decision support, knowledge representation and management in medicine. Yearb Med Inform 2006;45:72–80 [PubMed] [Google Scholar]
- 23.Ohno-Machado L, et al. The guideline interchange format: A model for representing guidelines. J Am Med Inform Assoc 1998;5:357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Arden Syntax for Medical Logic Systems Version 2.7. ANSI/HL7 Arden 2.7. Ann Arbor, MI: Health Level Seven International, 2008 [Google Scholar]