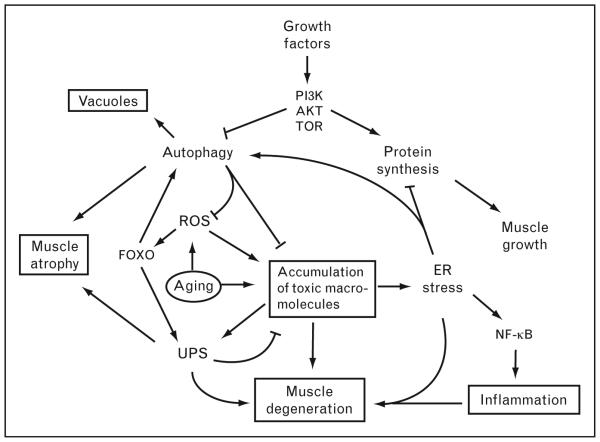
Figure 1. Model for pathogenesis of sporadic inclusion body myositis – disruption of protein homeostasis.
This model shows an interrelation of pathological hallmarks in sporadic inclusion body myositis (sIBM) including vacuoles, muscle atrophy, degeneration, accumulation of protein aggregates, and inflammation (shown in boxes). The consequences of aging (circled as the ‘initiating’ factor) include reactive oxygen species (ROS) and accumulation of ubiquitinated protein aggregates, degenerating mitochondria, and other macromolecules. In this model, these accumulations are both directly toxic to myofibers and also induce inflammation via induction of NF-κB. Protein aggregates are normally degraded via the ubiquitin–proteasome system (UPS) and autophagy, both of which are induced by the transcription factor FOXO. Growth factors such as IGF-1 stimulate protein synthesis and inhibit autophagy via the PI3K/Akt/TOR pathway to enable muscle growth. Disruption of autophagolysosome degradation may lead to accumulation of these structures as ‘rimmed vacuoles’ in sIBM patients. Thus, autophagy may be tightly regulated in muscle cells such that it is induced during cellular stress to allow degradation of toxic aggregates and inhibited during cell growth to allow synthesis of structural proteins.