Fig. 3. Mutation of Y520 and Y667 result in increased delivery of prestin to the apical surface of MDCK cells.
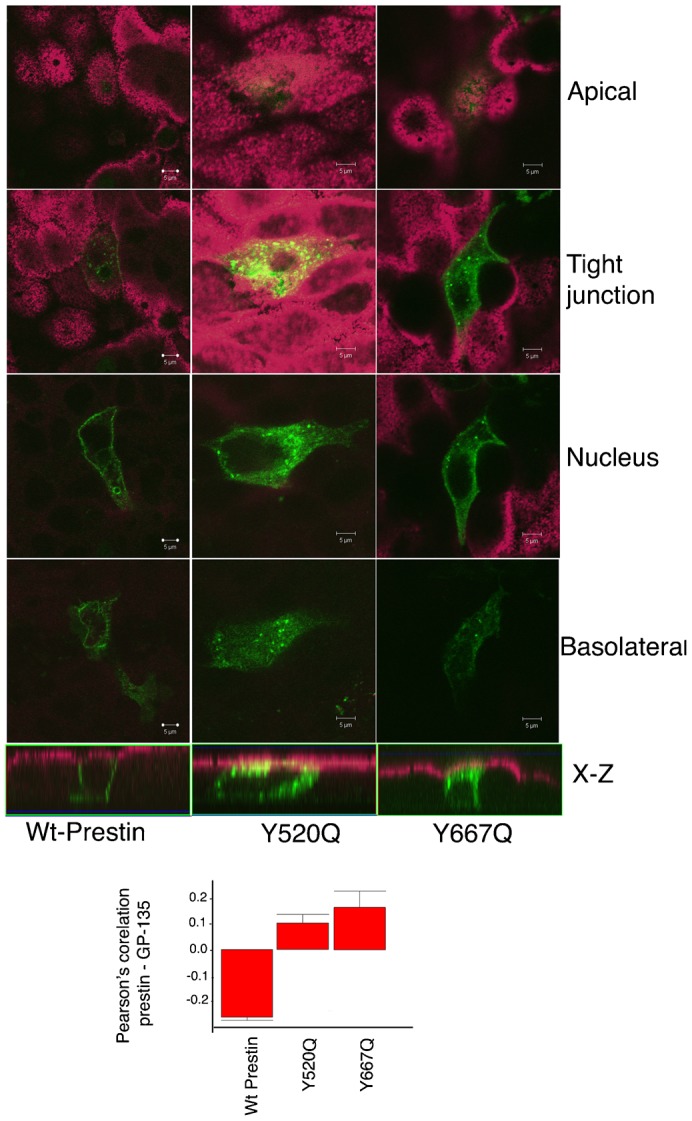
MDCK cells transiently transfected with wt prestin YFP and the two constructs Y520Q prestin YFP, and Y667Q prestin YFP were fixed at 36 hours and stained with antibody to the apical marker GP130 (podohexin). There is significant apical targeting of Y520Q and Y667Q evident in the serial X-Y sections along the z axis and the corresponding X-Z sections at the bottom. The bottom panel shows Pearson's correlation of prestin YFP and GP130 confirming absent apical targeting of the wild type construct and apical targeting of Y520Q and Y667Q. The mean Pearson's correlation values were: wt −0.027 (+/−0.011 SE, n = 7); Y520Q 0.105 (+/−0.035 SE, n = 5); Y667Q 0.17 (+/−0.063 SE, n = 11). The differences between wt prestin and Y520Q and wt prestin and Y667Q were significant. A one-way ANOVA (parametric) yielded a p value of <0.01 between wt prestin and Y520Q, and a p value of <0.001 between wt prestin and Y667Q. The scale bar is 5 microns.
