Fig. 5. The phenol ring in the tyrosine residue is critical for the targeting of prestin to the basolateral surface of MDCK cells.
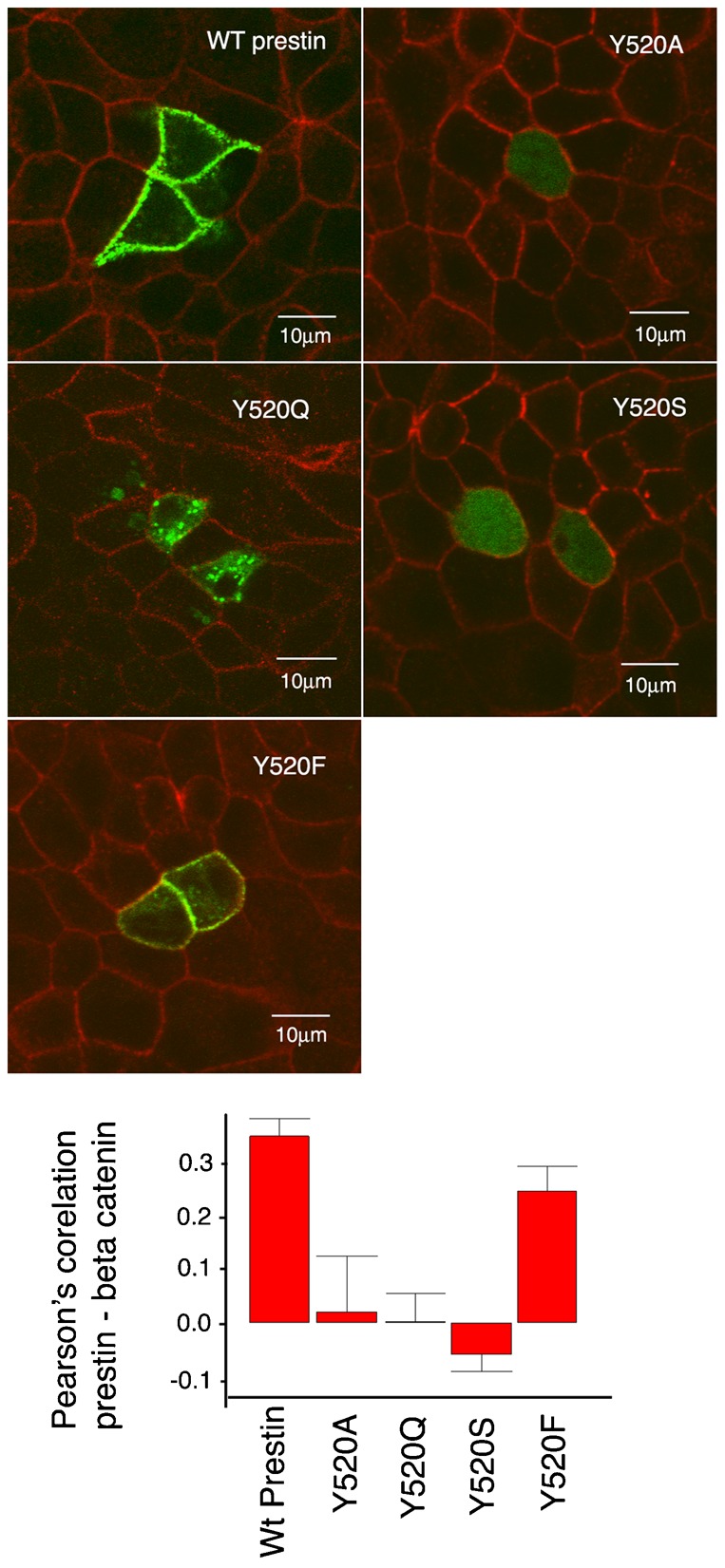
MDCK cells transiently transfected with prestin YFP and the mutations Y520Q, Y520A, Y520S and Y520F were fixed 36 hours after transfection and imaged by confocal microscopy. The basolateral wall of the cell was visualized by immunostaining with anti-β-catenin antibody. The mutations Y520A, Y520Q, and Y520S failed to target the basolateral surface of the cell, while Y520F was targeted to the basolateral surface of the cell. Pearson's correlation between prestin YFP and β–catenin confirm the observed co-localization of wild type prestin-YFP with β-catenin and Y520F with β-catenin. The mean Pearson's correlation values were: wt prestin 0.34 (+/−0.033 SE, n = 8); Y520A 0.019 (+/−0.10 SE, n = 5); Y520Q 0.002 (+/−0.052 SE, n = 5); Y520S −0.05 (+/−0.03, n = 6); Y520F 0.25 (+/−0.045 SE, n = 6). A one way ANOVA revealed significant differences between wt prestin and Y520A (P<0.01), wt prestin and Y520Q (P<0.001), and wt prestin and Y520S (P<0.001). The differences between wt prestin and Y520F were not significant. The scale bar is 10 microns.
