Fig. 5. FActin distribution and cellular morphology in control, netrin and fra mutant embryos.
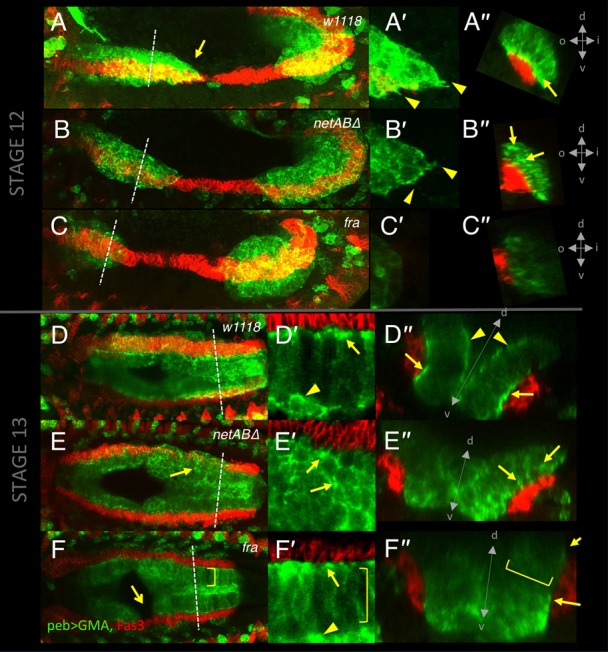
Stage 12 (A–C) and 13 (D–F) embryos immunostained for Fas3 (red) and GFP (green) in which the FActin marker GFP-MoeABD is expressed by the pebbled-GAL4 driver, in control (A,D), netABΔ (B,E) and fra3/Df(2R)BSC880 (C,F) mutant embryos. A′–C′ show the migrating front of the anterior midgut. A″–F″ show cross-sections at dotted lines in A–F. D′–F′ show a magnified image of the nascent epithelium in the posterior half of the midgut. (A) At mid stage 12 midgut cells from the anterior and posterior primordia are moving together (n = 8). Cells form a streamlined wedge shape (arrow), and extend protrusions (A′, arrowheads). FActin is enriched basally at the point of contact with the VM (A″, arrow). (B) In netABΔ mutants the overall shape of the migrating anterior midgut primordium is similar, and protrusions are evident (B′). Patches of FActin enrichment are present (arrows) but are not polarised to the basal side (B″) (n = 19). (C) In fra mutants, the midgut has a smoother profile (C′), basal polarisation is not clear, and patches of FActin enrichments are less obvious (C″) (n = 6). (D) Control embryo at stage 13. The midgut cells have organised themselves into a columnar monolayer. Due to variable GAL4 expression levels individual cells can be distinguished, extending from the VM through to the AMP cells on the apical surface of the epithelium (arrowhead). FActin is still basally polarised (D′,D″, arrows), though there is also some enrichment at the apical surface (D″, arrowhead) (n = 5). (E) In netABΔ mutants the columnar arrangement is less apparent (E′). FActin patches are prevalent (arrows) but located around cell bodies, and not polarised to the basal surface (E″) (n = 11). (F) fra mutant, in which a gap is still evident (arrow). The epithelium is closer to wild type (F′), and basal polarisation of FActin is greatly reduced (F″, arrow) (n = 5). FActin patches seen on lateral membranes in netABΔ embryos are missing (F,F′,F″ brackets). A″–F″ (d = dorsal, v = ventral, i = inside, o = outside).
