Abstract
Objective
Previous reports of the RAPID-axSpA trial (NCT01087762) described the efficacy and safety of certolizumab pegol (CZP) over 24 weeks in patients with axial spondyloarthritis (SpA), including ankylosing spondylitis (AS) and nonradiographic axial SpA. We report efficacy and safety data up to week 96 of the study.
Methods
The RAPID-axSpA trial is double-blind and placebo-controlled to week 24, dose-blind to week 48, and open-label to week 204. Outcome variables included Assessment of SpondyloArthritis international Society criteria for 20% and 40% improvement in disease activity (ASAS20/40), ASAS partial remission responses (analyzed by nonresponder imputation), AS Disease Activity Score (ASDAS), ASDAS inactive disease, ASDAS major improvement, Bath AS Disease Activity Index (BASDAI), Bath AS Functional Index (BASFI), and Bath AS Metrology Index (BASMI) linear score (analyzed by the last observation carried forward method). Safety data were collected for patients treated with ≥1 dose of CZP.
Results
Of the 325 patients who were randomized, 218 received CZP from week 0. Of these, 93% completed week 24, 88% completed week 48, and 80% completed week 96. Improvements in ASAS responses were maintained to week 96 (for ASAS20, 67.4%, 72.0%, and 62.8% at weeks 24, 48, and 96, respectively), as well as improvements in ASDAS, BASDAI (mean score 3.3, 3.1, and 3.0 at weeks 24, 48, and 96, respectively), BASFI, and BASMI linear score. Comparable improvements were observed with both dosing regimens (200 mg every 2 weeks or 400 mg every 4 weeks) and in patients with AS and those with nonradiographic axial SpA. In the safety set, adverse events occurred in 279 patients (88.6%) and serious adverse events in 41 (13.0%). No deaths or malignancies were reported.
Conclusion
Clinical improvements to week 24 in both CZP dosing regimens were sustained to week 96. Similar sustained improvements were observed in AS and nonradiographic axial SpA subpopulations. The safety profile was consistent with previous reports from RAPID-axSpA, with no new safety signals observed with longer exposure.
Axial spondyloarthritis (SpA) is a chronic inflammatory disease primarily characterized by inflammation of the sacroiliac (SI) joints and spine. Axial SpA encompasses a spectrum of disease, including ankylosing spondylitis (AS) (1) and axial SpA without radiographic evidence of AS (nonradiographic axial SpA) (2). There are often long delays between symptom onset and diagnosis, since back pain from axial SpA can be difficult to distinguish from more common causes of back pain. Historically, diagnosis has been challenging in patients with nonradiographic axial SpA because of the lack of definitive structural changes on SI joint radiographs. Nevertheless, the burden of disease is similar across the axial SpA spectrum (3), and patients with AS and nonradiographic axial SpA often suffer for many years before a diagnosis is made.
Due to the chronic nature of axial SpA, treatments must be tolerable and efficacious in controlling the signs and symptoms of disease over the long term. Current pharmacologic treatments are limited to either nonsteroidal antiinflammatory drugs (NSAIDs) or a limited number of anti–tumor necrosis factor (anti-TNF) drugs (4); hence, there is a substantial need for additional treatment options in this disease area. Furthermore, treatment options are especially limited for patients with nonradiographic axial SpA, with only 3 anti-TNF agents licensed for the treatment of nonradiographic axial SpA in the European Union (5–7). The long-term safety and efficacy data available for axial SpA treatment are predominantly focused on the AS subpopulation (8–11), with limited data available in the broader population of patients with axial SpA.
RAPID-axSpA is the first trial to present data on the efficacy of an anti-TNF agent across the broad spectrum of patients with active axial SpA as defined by the Assessment of SpondyloArthritis international Society (ASAS) criteria (2), including both patients with AS and those with nonradiographic axial SpA. In this trial, it was shown that certolizumab pegol (CZP), a PEGylated Fc-free anti-TNF agent, rapidly reduced the signs and symptoms of axial SpA in the broad population of patients with axial SpA over 24 weeks of treatment (12,13).
Here we present the first long-term data directly comparing outcomes in patients with AS and those with nonradiographic axial SpA, and we report the long-term efficacy and safety data for 2 CZP dosing regimens (200 mg every 2 weeks and 400 mg every 4 weeks) from the dose-blind (week 24–48) and early open-label (week 48–96) treatment periods of the RAPID-axSpA trial.
Patients and Methods
Patients
Detailed inclusion and exclusion criteria for the RAPID-axSpA trial have been reported previously (12). Eligible patients had active disease (Bath AS Disease Activity Index [BASDAI] [14] ≥4 and spinal pain ≥4), had an inadequate response to ≥1 NSAID, and fulfilled the ASAS criteria for adult-onset axial SpA (2). The study included patients with AS (>50% of patients) and patients with nonradiographic axial SpA (no definitive sacroiliitis on radiography), as defined by the modified New York criteria (1). Further exclusion criteria have been described previously (12).
Trial design
Treatment procedures
The RAPID-axSpA trial (http://ClinicalTrials.gov identifier: NCT01087762) is a phase III, multicenter trial of treatment of axial SpA, which is double-blind and placebo-controlled to week 24, dose-blind to week 48, and an open-label extension to week 204. Here we report the outcomes of the dose-blind (to week 48) and early open-label extension (to week 96) treatment periods. Patients were randomized 1:1:1 to receive placebo or to receive CZP 400 mg at weeks 0, 2, and 4 (loading dose) followed by either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks. Patients originally randomized to receive CZP in the double-blind phase continued to receive their assigned dose in the dose-blind phase and open-label extension. Patients randomized to receive placebo who were nonresponders according to the ASAS criteria for 20% improvement (ASAS20) (15) at both weeks 14 and 16 or patients randomized to receive placebo who completed the 24-week double-blind phase entered the dose-blind phase and were rerandomized 1:1 to receive CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks following the CZP loading dose (Figure 1A).
Figure 1.
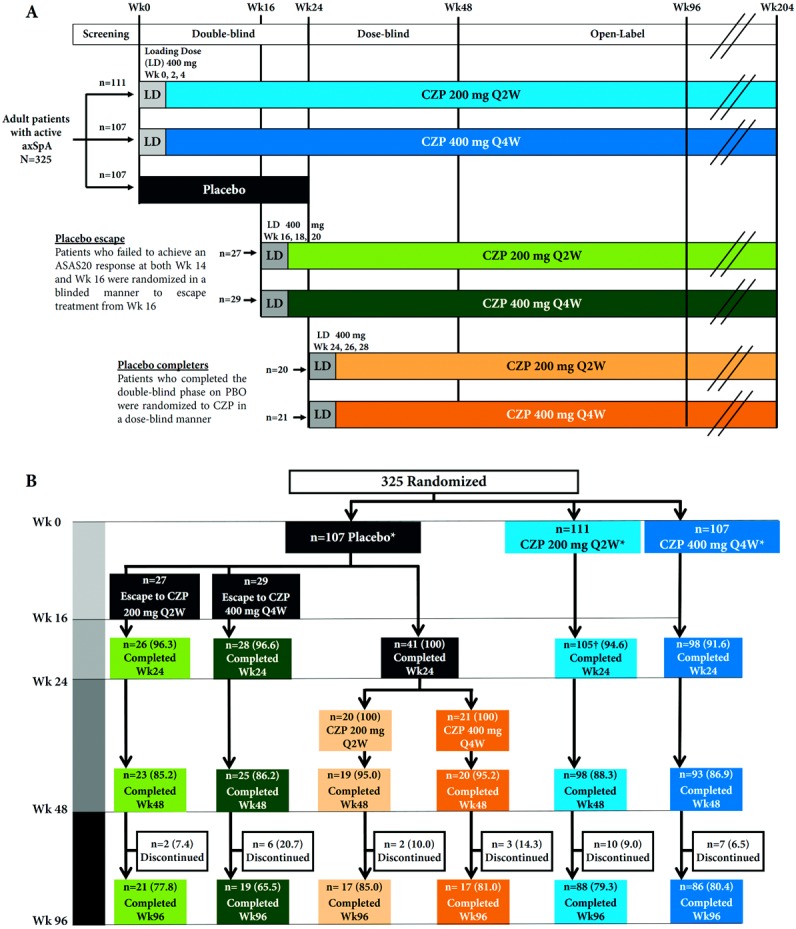
A, RAPID-axSpA trial design: certolizumab pegol (CZP) in active axial spondyloarthritis. B, Patient disposition in the RAPID-axSpA trial to week 96. * All patients received allocated treatment. † One patient did not enroll in the dose-blind trial period. Values are the number (%) of patients. Q2W = every 2 weeks; Q4W = every 4 weeks; ASAS20 = Assessment of SpondyloArthritis international Society criteria for 20% improvement in disease activity; PBO = placebo.
At all study sites, all investigators and other health care professionals involved with safety or efficacy assessments were completely blinded with regard to the study medications. Due to differences in the presentation and viscosity of CZP and placebo, all study treatments (CZP and placebo) were administered by dedicated, unblinded, trained study center personnel who had no other involvement in the study, to maintain study blinding.
Evaluations
The primary end point of RAPID-axSpA (ASAS20 response at week 12) has been reported previously (12). Efficacy outcomes reported here include self-reported assessments for back pain and disease activity (0–10 numeric rating scales), as well as health-related quality of life, which was assessed using the Short Form 36 (SF-36) mental and physical component summaries (16). Serum C-reactive protein (CRP) levels were assessed as an objective measure of inflammation. Spinal mobility and functionality were assessed using the Bath AS Metrology Index (BASMI) linear score (17) and Bath AS Functional Index (BASFI) (18), respectively.
Efficacy results to week 96 are presented using composite end points validated for use by ASAS (19): ASAS20, ASAS40, ASAS partial remission, and ASAS5/6 criteria. The BASDAI and AS Disease Activity Score (ASDAS) were used to assess disease activity (20). In addition to absolute scores, we report the proportion of patients in whom a ≥50% improvement in the BASDAI from baseline (BASDAI 50), ASDAS major improvement, and ASDAS inactive disease criteria were achieved. ASDAS major improvement was defined as a reduction in ASDAS of ≥2 from baseline, and ASDAS inactive disease was defined as an ASDAS of <1.3.
The long-term safety of CZP was investigated through analysis of adverse events (AEs) at every study visit to week 96. For laboratory analyses of plasma concentrations of anti-CZP antibodies, levels of >2.4 units/ml at ≥1 study visit were considered positive (21).
In an attempt to identify a potential increase in the risk of tuberculosis infection with CZP treatment, the sponsor required that all patients have a systematic purified protein derivative (PPD) test (and/or interferon-γ–release assay [IGRA]) at weeks 48 and 96, regardless of risk of tuberculosis (signs, symptoms, or close contact with a tuberculosis patient). All cases of PPD ≥5 mm or suspected tuberculosis were reviewed post hoc by independent experts. Active tuberculosis was confirmed according to the World Health Organization criteria for tuberculosis.
Statistical analysis
The majority of efficacy outcomes are presented for all patients randomized to receive either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks at baseline (randomized set), although selected data are also shown for patients who were rerandomized from placebo to either of the CZP dose regimens at week 16 or 24. Response rates (%) were calculated using nonresponder imputation (NRI), and quantitative assessments are shown as arithmetic means and use last observation carried forward imputation. Observed data are also shown for patients completing week 96. Analyses are presented in the overall axial SpA population and for the AS and nonradiographic axial SpA subpopulations. Data are shown with no inferential statistics. Safety data are presented for all patients treated with ≥1 dose of CZP at any stage of the 96-week trial period (safety set). Placebo-treated patients rerandomized to receive CZP were included from the date of their first CZP injection. Geometric means for CZP plasma concentrations were analyzed for all subjects from the safety set who had valid pharmacokinetic assessments.
Results
Patient disposition and baseline characteristics
Of a total of 325 patients who were randomized, 218 received CZP (200 mg every 2 weeks or 400 mg every 4 weeks) from week 0 (randomized set). Of these patients, 203 (93%) remained in the study to week 24, 191 (88%) to week 48, and 174 (80%) to week 96 (Figure 1B). Between week 24 and week 48, 3 patients (1.4%) withdrew due to an AE, 3 (1.4%) due to lack of efficacy, and 5 (2.3%) due to other reasons. Between week 48 and week 96, 11 patients (5.0%) withdrew due to an AE, 2 (0.9%) due to lack of efficacy, and 4 (1.8%) due to other reasons. The numbers of patients who withdrew from the study were similar in both the AS and nonradiographic axial SpA subpopulations.
Of the 315 patients who received any dose of CZP, 35 (11.1%) experienced an AE leading to discontinuation of study drug. Of these patients, 6 (1.9%) withdrew due to infection (the most frequent cause). Of note, 14 patients (4.4%) were withdrawn by the sponsor due to their having a PPD tuberculin skin test >5 mm (and one positive IGRA test). According to study protocol, the PPD test was performed in all patients, including those who were asymptomatic or had no additional risk factors for tuberculosis.
Of the 107 patients originally randomized to receive placebo, 56 (52.3%) entered an early escape phase (according to study protocol) and were rerandomized in a double-blind manner at week 16 to receive CZP 200 mg every 2 weeks (n = 27) or CZP 400 mg every 4 weeks (n = 29) following a CZP loading dose (Figure 1B). At week 24, 41 placebo completers were rerandomized to receive CZP 200 mg every 2 weeks (n = 20) or CZP 400 mg every 4 weeks (n = 21) following a CZP loading dose (Figure 1B). The proportions of placebo completers were similar between the 2 subpopulations: of the 41 patients, 18 (43.9%) had nonradiographic axial SpA and 23 (56.1%) had AS.
Disease characteristics were similar between all populations for those patients randomized to receive CZP at baseline (Table1). It was noted that placebo-treated patients who demonstrated a clinical response at week 14 or 16 and continued to receive placebo until week 24 had less severe disease activity at baseline compared with placebo-treated patients who did not respond clinically (and who therefore withdrew from the placebo arm at week 16) (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002.art.38973/abstract).
Table 1.
Baseline characteristics of the patients randomized to receive CZP*
| All patients with axial SpA (n = 218) | Patients with AS (n = 121) | Patients with nonradiographic axial SpA (n = 97) | |
|---|---|---|---|
| Age, mean ± SD years | 39.5 ± 11.6 | 41.4 ± 11.1 | 37.1 ± 11.8 |
| Male sex | 135 (61.9) | 88 (72.7) | 47 (48.5) |
| Symptom duration, median (minimum, maximum) years | 7.8 (0.3, 44.8) | 8.8 (0.3, 44.8) | 5.9 (0.3, 34.2) |
| Symptom duration <5 years | 84 (38.5) | 41 (33.9) | 43 (44.3) |
| CRP, median mg/liter | 12.5 | 14.0 | 11.0 |
| >ULN (7.9 mg/liter) | 146 (67.0) | 85 (70.2) | 61 (62.9) |
| ≥15 mg/liter | 80 (36.7) | 49 (40.5) | 31 (32.0) |
| BASDAI, mean ± SD | 6.4 ± 1.5 | 6.4 ± 1.5 | 6.6 ± 1.5 |
| BASFI, mean ± SD | 5.3 ± 2.3 | 5.6 ± 2.3 | 5.0 ± 2.3 |
| BASMI, mean ± SD | 3.8 ± 1.7 | 4.2 ± 1.7 | 3.2 ± 1.5 |
| ASDAS, mean ± SD | 3.8 ± 0.9 | 3.9 ± 0.9 | 3.8 ± 0.8 |
| Peripheral arthritis† | 76 (34.9) | 42 (34.7) | 34 (35.1) |
| Enthesitis‡ | 148 (67.9) | 78 (64.5) | 70 (72.2) |
| Extraspinal features of axial SpA§ | |||
| Heel enthesitis | 72 (33.0) | 41 (33.9) | 31 (32.0) |
| Uveitis | 38 (17.4) | 20 (16.5) | 18 (18.6) |
| Psoriasis | 13 (6.0) | 5 (4.1) | 8 (8.2) |
| Crohn's disease/ulcerative colitis | 10 (4.6) | 6 (5.0) | 4 (4.1) |
Except where indicated otherwise, values are the no. (%). Results are shown for the randomized set (all patients randomized at baseline to receive either certolizumab pegol [CZP] 200 mg every 2 weeks or CZP 400 mg every 4 weeks). SpA = spondyloarthritis; AS = ankylosing spondylitis; CRP = C-reactive protein; ULN = upper limit of normal; BASDAI = Bath AS Disease Activity Index; BASFI = Bath AS Functional Index; BASMI = Bath AS Metrology Index; ASDAS = AS Disease Activity Score.
Defined as at least 1 swollen joint in a 44-joint assessment.
Defined as a Maastricht AS Enthesitis Score of >0.
Either patient history or current diagnosis, defined by the Assessment of SpondyloArthritis international Society classification criteria screening assessment.
Efficacy outcomes
Improvements from baseline to week 24 in disease activity (ASDAS and BASDAI), spinal mobility (BASMI), and spinal function (BASFI) were maintained throughout the dose-blind trial period to week 48 and the open-label extension to week 96 (Figures 2A–C and 3A–C) (see Supplementary Figure 1 and Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38973/abstract). Improvements in the BASDAI 50 were maintained from week 24 to week 96 in patients receiving CZP 200 mg every 2 weeks (50.5% at week 24 and 46.8% at week 96) and those receiving CZP 400 mg every 4 weeks (54.2% at week 24 and 48.6% at week 96). For some outcomes, such as ASDAS major improvement and ASDAS inactive disease, a small improvement was seen between week 24 and week 96 (Figures 2B and C and Supplementary Table 2).
Figure 2.
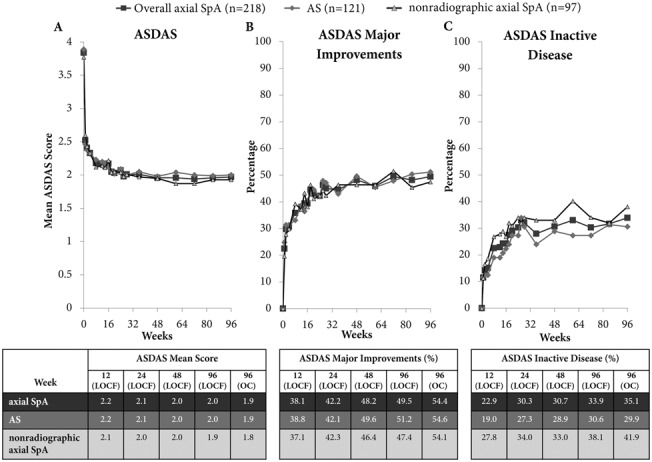
Mean Ankylosing Spondylitis Disease Activity Score (ASDAS) (A), percentage of patients in whom ASDAS major improvement was achieved (B), and percentage of patients with ASDAS inactive disease (C) to week 96 of certolizumab pegol (CZP) treatment in patients with axial spondyloarthritis (axSpA), patients with AS, and patients with nonradiographic axial SpA. Graphs show last observation carried forward (LOCF) data. Results are reported for the randomized set (all patients randomized at baseline to receive either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks). 96 (OC) = observed case data for week 96 (n = 171 patients with axial SpA; n = 97 patients with AS; n = 74 patients with nonradiographic axial SpA).
Figure 3.
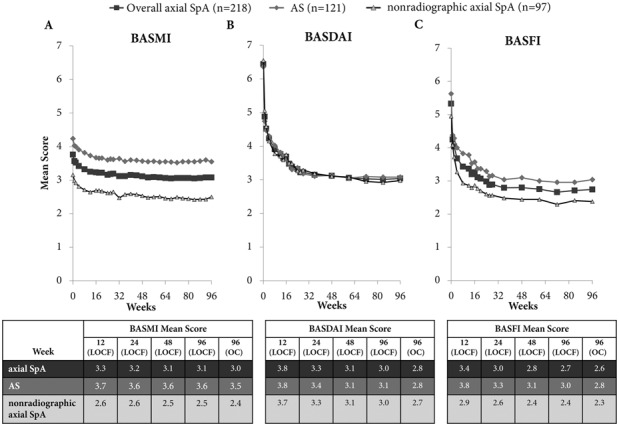
Mean Bath Ankylosing Spondylitis Metrology Index (BASMI) linear score (A), mean Bath AS Disease Activity Index (BASDAI) score (B), and mean Bath AS Functional Index (BASFI) score (C) to week 96 of certolizumab pegol (CZP) treatment in patients with axial spondyloarthritis (axSpA), patients with AS, and patients with nonradiographic axial SpA. Graphs show last observation carried forward (LOCF) data. Results are reported for the randomized set (all patients randomized at baseline to receive either CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks). 96 (OC) = observed case data for week 96 (n = 171 patients with axial SpA; n = 97 patients with AS; n = 74 patients with nonradiographic axial SpA).
Similarly, improvements in ASAS20, ASAS40, and ASAS partial remission responses, which were seen for both CZP 200 mg every 2 weeks and CZP 400 mg every 4 weeks in the 24-week double-blind phase, were maintained through the dose-blind phase to week 48 and to week 96 in the open-label extension (Figure 4A). Improvements were maintained across both the AS and nonradiographic axial SpA subpopulations (Figure 4B). A comparison of imputed and observed ASAS response rates in this study demonstrated the more conservative nature of NRI (Figure 4), with an ASAS20 response achieved in 82.0% of patients who completed the study to week 96 compared with 62.8% when data were imputed using NRI. Improvements in ASAS5/6 response seen in the double-blind study period were maintained in the dose-blind and open-label periods (41.3% for week 24, 41.7% for week 48, and 42.2% for week 96 for the overall axial SpA population [NRI]) (Supplementary Table 2). Improvements in nocturnal back pain and health-related quality of life (AS Quality of Life (22), SF-36 mental component summary, and SF-36 physical component summary) were maintained to week 96 of CZP treatment (see Supplementary Table 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38973/abstract).
Figure 4.
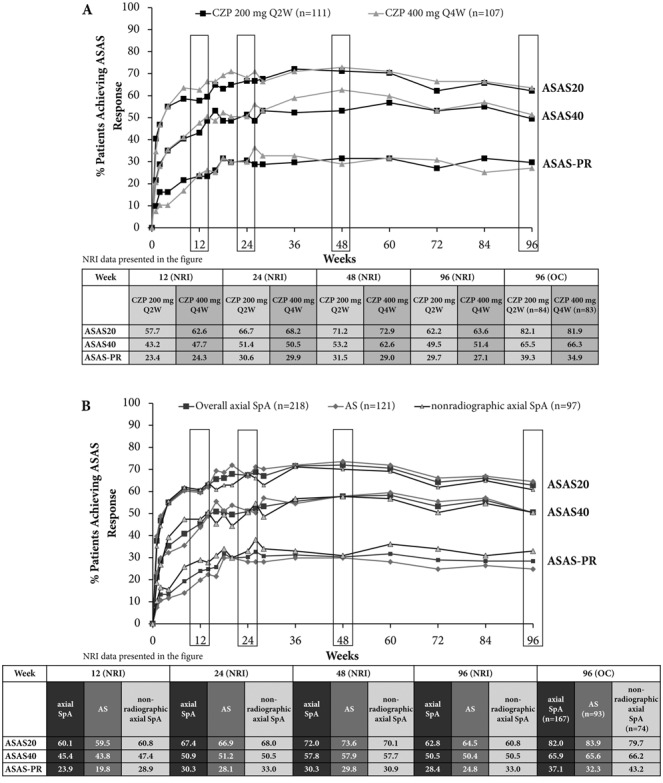
Percentage of patients in whom Assessment of SpondyloArthritis international Society criteria for 20% improvement in disease activity (ASAS20), ASAS40, and ASAS partial remission response (ASAS-PR) were achieved to week 96 of certolizumab pegol (CZP) treatment. A, ASAS responses in the overall population of patients with axial spondyloarthritis (axSpA) separated by dose. B, ASAS responses in the overall population of patients with axial SpA, ankylosing spondylitis (AS), or nonradiographic axial SpA populations, with all doses combined. Graphs show nonresponder imputation (NRI) data. Results are reported for the randomized set (all patients randomized at baseline to receive either CZP 200 mg every 2 weeks [Q2W] or CZP 400 mg every 4 weeks). 96 (OC) = observed case data for week 96.
Similar efficacy responses were seen in patients with prior anti-TNF exposure (n = 26) and those without prior anti-TNF exposure (n = 192); improvements were maintained to week 96 in both response measures and continuous outcomes. At week 96, ASAS40 responses were 50.0% (with prior anti-TNF exposure) and 50.5% (without prior anti-TNF exposure) and BASDAI change from baseline scores were −3.5 (with prior anti-TNF exposure) and −3.4 (without prior anti-TNF exposure). However, these results should be interpreted with caution given the low patient numbers in these analyses.
Patients originally randomized to receive placebo who either escaped early at week 16 or completed the double-blind phase to week 24 and were subsequently rerandomized to receive CZP treatment (CZP 200 mg every 2 weeks or CZP 400 mg every 4 weeks) had improvements in both ASAS20 response and ASDAS score following their switch to CZP treatment, which were maintained to week 96 of the study (see Supplementary Figure 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.38973/abstract).
We also looked at the baseline Spondyloarthritis Research Consortium of Canada (SPARCC) magnetic resonance imaging (MRI) index for SI joint inflammation (23) and the CRP level as potential predictors of response to CZP. These data indicated a possible association between higher baseline CRP levels or higher baseline SPARCC scores and a greater chance of achieving ASDAS major improvement or an ASAS40 response, a benefit that was maintained to week 96 (data not shown).
Safety
The safety set consisted of 315 patients who received ≥1 dose of CZP at any stage of the 96-week trial period. The total exposure to CZP was 486 patient-years. AEs occurred in 279 patients (88.6%; event rate/100 patient-years = 360.3) and were predominantly mild (74.9%) or moderate (59.4%) in nature (Table2). Serious AEs occurred in 41 patients (13.0%; event rate/100 patient-years = 10.9). Serious infections occurred in 12 patients (3.8%; event rate/100 patient-years = 2.7). One case of active tuberculosis infection was identified (0.3%; event rate/100 patient-years = 0.2). No new safety signals were observed, and no deaths, malignancies, or cases of demyelinating disease were reported during the 96-week trial period.
Table 2.
AEs to week 96 of the RAPID-axSpA trial*
| CZP 200 mg every 2 weeks (n = 111) |
CZP 400 mg every 4 weeks (n = 107) |
All CZP (n = 315) |
||||
|---|---|---|---|---|---|---|
| No. (%) | Event rate/100 patient-years | No. (%) | Event rate/100 patient-years | No. (%) | Event rate/100 patient-years | |
| Any AE | 104 (93.7) | 376.5 | 92 (86.0) | 352.5 | 279 (88.6) | 360.3 |
| Serious AEs | 13 (11.7) | 8.2 | 14 (13.1) | 9.1 | 41 (13.0) | 10.9 |
| Most frequent serious AEs† | ||||||
| Gastrointestinal disorders | 0 | – | 3 (2.8) | 1.7 | 4 (1.3) | 0.8 |
| Infections and infestations | 4 (3.6) | 2.7 | 2 (1.9) | 1.1 | 12 (3.8) | 2.7 |
| Injury, poisoning, and procedural complications | 1 (0.9) | 0.5 | 1 (0.9) | 0.6 | 4 (1.3) | 1.0 |
| Musculoskeletal and connective tissue disorders | 1 (0.9) | 0.5 | 1 (0.9) | 0.6 | 4 (1.3) | 0.8 |
| AEs by intensity | ||||||
| Mild | 89 (80.2) | – | 78 (72.9) | – | 236 (74.9) | – |
| Moderate | 69 (62.2) | – | 62 (57.9) | – | 187 (59.4) | – |
| Severe | 8 (7.2) | – | 9 (8.4) | – | 31 (9.8) | – |
| Drug-related AEs | 56 (50.5) | – | 52 (48.6) | – | 148 (47.0) | – |
| Deaths | 0 | – | 0 | – | 0 | – |
Results are reported for the safety set (all patients treated with ≥1 dose of certolizumab pegol [CZP] at any stage of the 96-week trial period). AEs = adverse events; axSpA = axial spondyloarthritis.
Occurring in >1% of the safety population by Medical Dictionary for Regulatory Activities System Organ Class.
Of the patients treated with CZP, 215 were tested for the presence of anti-CZP antibodies at week 96. Of these patients, 9 (4.2%) were observed to have developed anti-CZP antibodies. The proportion of patients developing anti-CZP antibodies was roughly similar in both CZP dosing regimens, with 2.7% and 5.8% of patients testing positive for anti-CZP antibodies at week 96 in the CZP 200 mg every 2 weeks and CZP 400 mg every 4 weeks treatment groups, respectively. The plasma concentration of CZP was lower in patients with anti-CZP antibodies than in those without (geometric mean 2.5 units/ml and 19.7 units/ml, respectively). Since the number of patients developing anti-CZP antibodies was relatively low, an investigation into efficacy in this subgroup of patients was not carried out.
Discussion
The rapid improvements observed over the first 24 weeks of the RAPID-axSpA trial in clinical measures of efficacy and patient-reported outcomes were maintained in both CZP dosing regimens and in both AS and nonradiographic axial SpA patients throughout the dose-blind treatment period (in which patients and investigators were blinded with regard to CZP dosing regimen to week 48) and the open-label extension period (week 48 to week 96). Indeed, for some outcome measures, slight improvements were observed between week 24 and week 96.
RAPID-axSpA is the first study to investigate the efficacy of an anti-TNF agent in the broad population of patients with axial SpA, including both those with AS and those with nonradiographic axial SpA. Baseline disease activity, as measured by BASDAI and ASDAS, was similar in the AS and nonradiographic axial SpA subpopulations. The similarities between these subpopulations in terms of disease characteristics and burden (24,25) underline the need for more treatment options to be available across the broader population of patients with axial SpA. The similarity in improvements observed across both patients with AS and those with nonradiographic axial SpA indicates that CZP is efficacious for the treatment of patients with axial SpA with objective signs of inflammation, independently of whether the patient has sufficient structural damage in the SI joints to meet the modified New York classification criteria for AS.
RAPID-axSpA is also the first study to report long-term efficacy data for patients with axial SpA, who were required to have CRP levels and/or evidence on MRI indicative of SpA for inclusion in the trial; these characteristics have been associated with a good response to anti-TNF treatment. The maintenance of response seen with continued CZP treatment to week 96 confirms its long-term efficacy in axial SpA patients with objective signs of inflammation. Additionally, the similar long-term reduction in disease activity for both CZP 200 mg every 2 weeks and CZP 400 mg every 4 weeks is important, since this may offer the treating physician more freedom to switch between the dosing regimens.
The ASAS20 scores observed over the first 96 weeks of the RAPID-axSpA trial were comparable to those observed over a similar time period with adalimumab treatment of patients with nonradiographic axial SpA (26) and with etanercept (10), adalimumab (11), infliximab (8), and golimumab (9) treatment of AS patients. However, care must be taken when interpreting intertrial comparisons, since different studies use different patient populations, trial designs, and data analysis methods. Here we reported ASAS20 response rates conservatively using NRI as well as observed data, according to recommendations for reporting long-term results (27). Consistent with previous reports of CZP in axial SpA (12), AEs were predominantly mild to moderate in nature, with 9.8% of AEs considered to be severe and with a serious AE event rate of 10.9/100 patient-years. There were no malignancies or deaths observed over the 96-week trial period and no new safety signals identified compared with previous reports of CZP.
The presence of anti-CZP antibodies was low in this trial, with only 9 of 215 patients testing positive at week 96 (4.2%). Due to the low numbers of anti-CZP–positive patients, an investigation into the impact of antibody levels on treatment efficacy was not carried out.
This study has a number of limitations, including the lack of a placebo arm beyond week 24, and limitations inherent to the unblinded nature of the open-label study period. Furthermore, no radiographic data are presented here, precluding any analysis of disease progression from nonradiographic axial SpA to AS.
In this study, the rapid improvements in clinical outcomes observed in axial SpA patients treated with CZP over 24 weeks were maintained through the dose-blind phase to week 48 and the open-label extension to week 96 in both the AS and nonradiographic axial SpA subpopulations. This maintenance of efficacy was observed when CZP was administered as either 200 mg every 2 weeks or 400 mg every 4 weeks. The safety profile of CZP in patients with axial SpA over a period of 96 weeks was comparable with that reported over shorter time periods and in other indications, with no new previously unreported safety signals occurring over the 96-week trial period.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sieper had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sieper, Deodhar, Hoepken.
Acquisition of data. Landewé, Rudwaleit, van der Heijde, Dougados, Mease, Braun, Deodhar, Kivitz, Walsh, Hoepken, Maksymowych.
Analysis and interpretation of data. Sieper, Landewé, Rudwaleit, van der Heijde, Dougados, Mease, Braun, Deodhar, Kivitz, Walsh, Hoepken, Nurminen, Maksymowych.
Role of the Study Sponsor
UCB Pharma sponsored the study and the development of the manuscript. In addition to content approval by the authors, UCB signed off on the manuscript following a full review to ensure that the publication did not contain any information which has the potential to damage the intellectual property of UCB.
Acknowledgments
The authors acknowledge Owen Davies and Marine Champsaur (UCB Pharma, Belgium) for critical review, Pritibha Singh (UCB Pharma, Germany) for statistical support, and Costello Medical Consulting, UK, for writing and editorial assistance, which was funded by UCB Pharma.
References
- 1.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–8. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 2.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 3.Mease PJ, Tubergen A, Deodhar A, Coteur G, Nurminen T, van der Heijde D. Comparing health-related quality of life across rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: analyses from certolizumab pegol clinical trial baseline data. Ann Rheum Dis. 2013;72(Suppl 3):A766–7. [abstract]. [Google Scholar]
- 4.Poddubnyy D. Axial spondyloarthritis: is there a treatment of choice? Ther Adv Musculoskelet Dis. 2013;5:45–54. doi: 10.1177/1759720X12468658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicines Agency (EMA) 2012. Annex 1: Summary of Product Characteristics (Humira). URL: http://www.ema.europa.eu/ema/
- 6.European Medicines Agency (EMA) 2013. Annex 1: Summary of Product Characteristics (Cimzia). URL: http://www.ema.europa.eu/ema/
- 7.European Medicines Agency (EMA) 2014. Annex 1: Summary of Product Characteristics (Enbrel). URL: http://www.ema.europa.eu/ema/
- 8.Braun J, Deodhar A, Dijkmans B, Geusens P, Sieper J, Williamson P Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis over a two-year period. Arthritis Rheum. 2008;59:1270–8. doi: 10.1002/art.24001. et al and the. [DOI] [PubMed] [Google Scholar]
- 9.Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis. 2012;71:661–7. doi: 10.1136/ard.2011.154799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JC, van der Heijde DM, Braun J, Dougados M, Clegg DO, Kivitz AJ. Efficacy and safety of up to 192 weeks of etanercept therapy in patients with ankylosing spondylitis. Ann Rheum Dis. 2008;67:346–52. doi: 10.1136/ard.2007.078139. Jr, et al. [DOI] [PubMed] [Google Scholar]
- 11.Van der Heijde D, Schiff MH, Sieper J, Kivitz AJ, Wong RL, Kupper H. Adalimumab effectiveness for the treatment of ankylosing spondylitis is maintained for up to 2 years: long-term results from the ATLAS trial. Ann Rheum Dis. 2009;68:922–9. doi: 10.1136/ard.2007.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landewe R, Braun J, Deodhar A, Dougados M, Maksymowych W, Mease P. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieper J, Kivitz A, van Tubergen A, Deodhar A, Coteur G, Woltering F. Rapid improvements in patient-reported outcomes with certolizumab pegol in patients with axial spondyloarthritis, including ankylosing spondylitis: 24-week results of RAPID-axSpA Study. Ann Rheum Dis. 2013;72(Suppl 3):A287. et al. [abstract]. [Google Scholar]
- 14.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 15.Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing Spondylitis Assessment Group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001;44:1876–86. doi: 10.1002/1529-0131(200108)44:8<1876::AID-ART326>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. Jr. [Google Scholar]
- 17.Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS): the Bath AS Metrology Index. J Rheumatol. 1994;21:1694–8. [PubMed] [Google Scholar]
- 18.Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21:2281–5. [PubMed] [Google Scholar]
- 19.Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):ii1–44. doi: 10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 20.Van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J Assessment of SpondyloArthritis international Society (ASAS) ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68:1811–8. doi: 10.1136/ard.2008.100826. et al, for the. [DOI] [PubMed] [Google Scholar]
- 21.Fleischmann R, Vencovsky J, van Vollenhoven RF, Borenstein D, Box J, Coteur G. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–11. doi: 10.1136/ard.2008.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helliwell PS, Doward L, Whalley D, Tennant A, McKenna S, Reynolds S. Psychometric and scaline properties of a new quality of life instrument specific to ankylosing spondylitis. Arthritis Rheum. 1999;42(Suppl):S72. et al. [abstract]. [Google Scholar]
- 23.Maksymowych WP, Inman RD, Salonen D, Dhillon SS, Williams M, Stone M. Spondyloarthritis Research Consortium of Canada Magnetic Resonance Imaging Index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum. 2005;53:703–9. doi: 10.1002/art.21445. [DOI] [PubMed] [Google Scholar]
- 24.Mease PJ, van Tubergen A, Deodhar A, Coteur G, Nurminen T, van der Heijde D. Comparing health-related quality of life across rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: analyses from certolizumab pegol clinical trial baseline data. Value Health. 2013;16:A570. [abstract].. URL: http://www.valueinhealthjournal.com/article/S1098-3015(13)03433-5/pdf. [Google Scholar]
- 25.Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker-Hermann E, Zeidler H. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60:717–27. doi: 10.1002/art.24483. [DOI] [PubMed] [Google Scholar]
- 26.Sieper J, van der Heijde D, Dougados M, Van den Bosch F, Goupille P, Sarkar S. Sustained efficacy of adalimumab in patients with non-radiographic axial spondyloarthritis: week 68 results from ABILITY 1. Ann Rheum Dis. 2012;71(Suppl 3):248. et al. [abstract]. [Google Scholar]
- 27.Papp KA, Fonjallaz P, Casset-Semanaz F, Krueger JG, Wittkowski KM. Analytical approaches to reporting long-term clinical trial data. Curr Med Res Opin. 2008;24:2001–8. doi: 10.1185/03007990802215315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


