Abstract
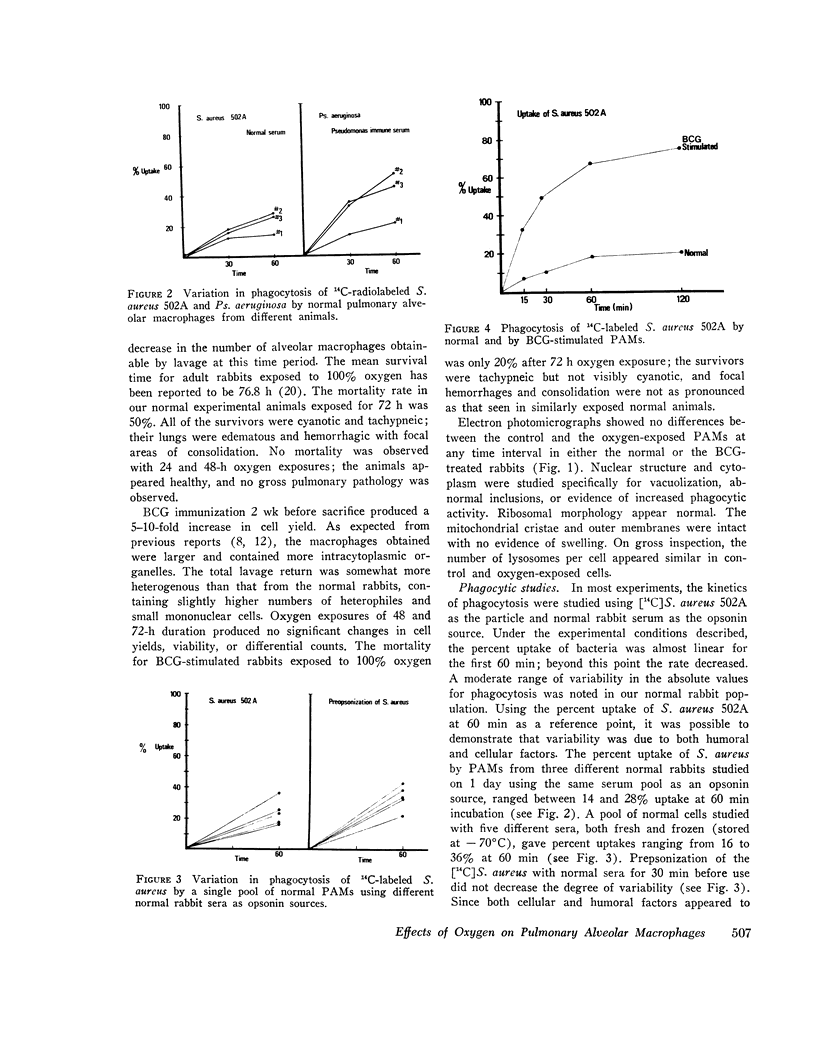
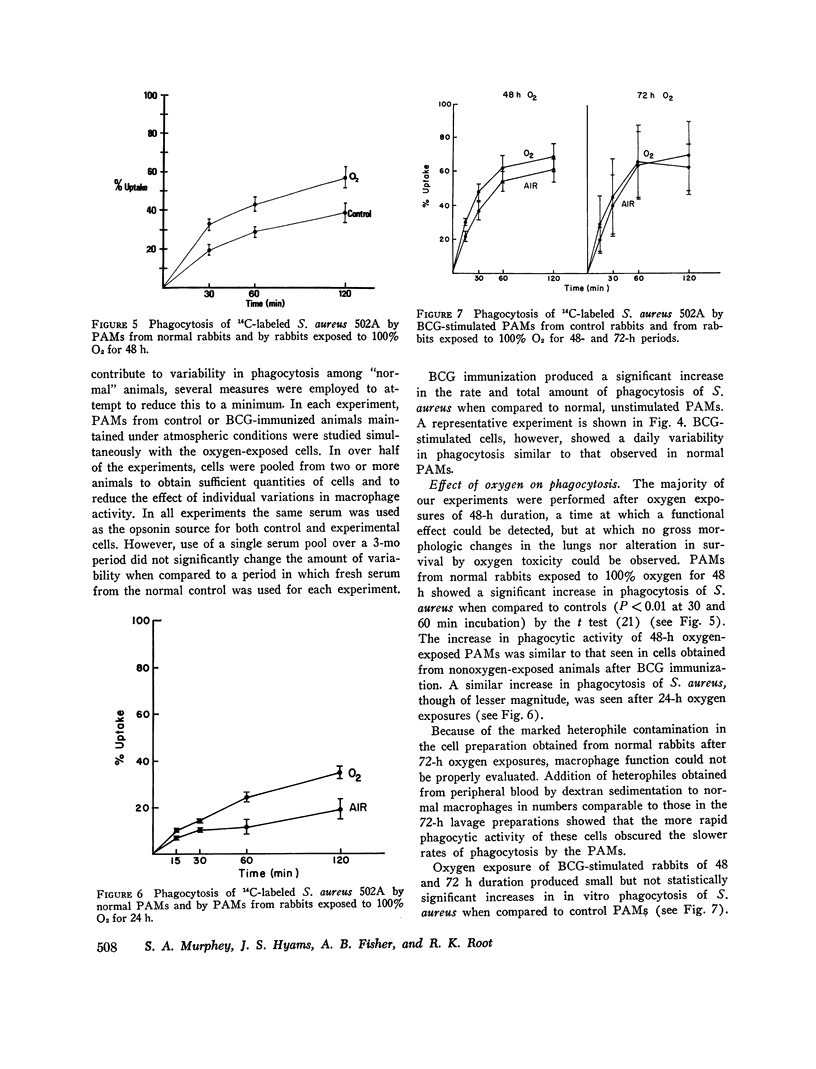
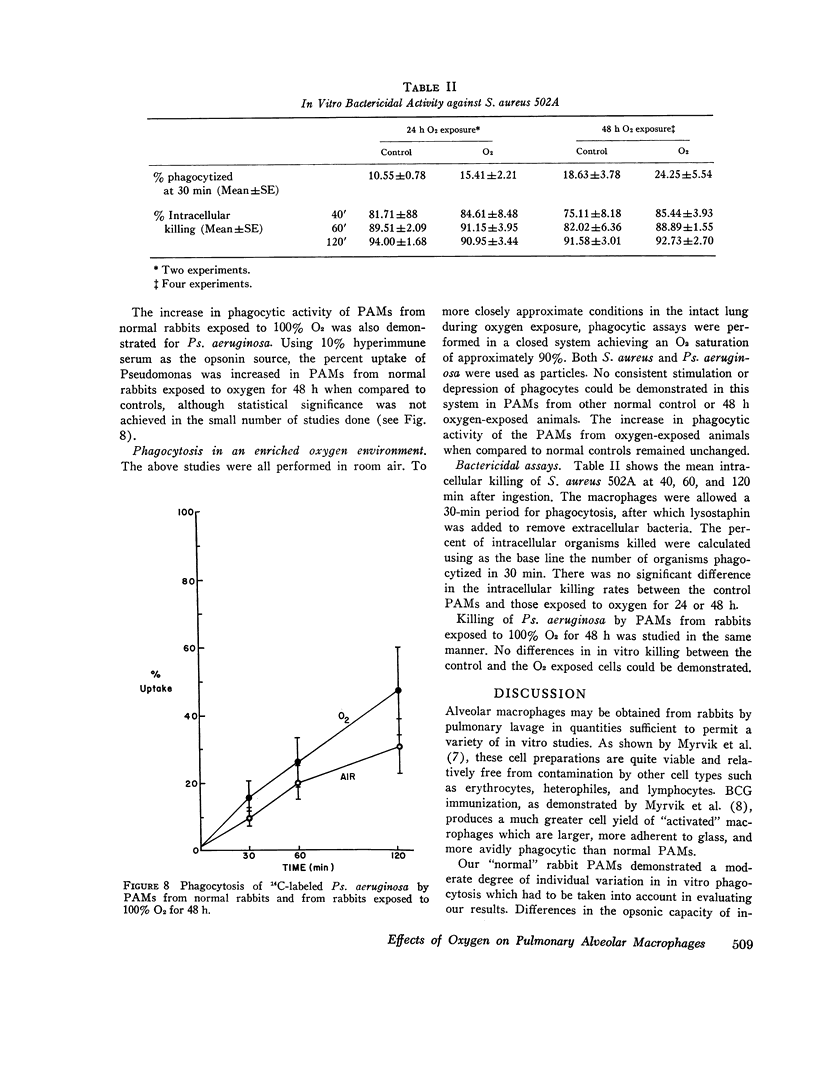
Bacterial infection may complicate pulmonary oxygen (O2) toxicity, and animals exposed to high O2 concentrations show depressed in vivo pulmonary bacterial inactivation. Therefore, in vitro studies were undertaken to define the mechanism by which O2 alters pulmonary antibacterial activity. Normal and BCG pretreated rabbits were exposed to 100% O2 for 24, 48, and 72-h periods. Pulmonary alveolar macrophages (PAM) were obtained from the experimental animals and from nonoxygen exposed controls by bronchopulmonary lavage. O2 exposure did not alter cell yield or morphology. PAMs were suspended in 10% serum-buffer, and phagocytosis of (14C)Staphylococcus aureus 502A and (14C)Pseudomonas aeruginosa was measured. Comparison of the precent uptake of the 14C-labeled S. aureus after a 60-min incubation period demonstrated that normal PAMs exposed to O2 for 48 h showed a statistically significant increase in phagocytosis when compared to their controls (43.5 vs. 29.2%). A similar, but smaller increase was seen after 24-h O2 exposures. 48 and 72-h O2 exposures produced no significant changes in phagocytosis in PAMs from BCG-stimulated rabbits. Normal PAMs also showed an increased phagocytosis of Ps. aeruginosa after 48-h oxygen exposure. No impairment of in vitro bactericidal activity against either S. aureus 502A or Ps. aeruginosa could be demonstrated in PAMs from normal rabbits exposed to O2 for 48 h. These results indicate that the in vitrophagocytic and bactericidal capacity of the rabbit PAM is relatively resistant to the toxic effects of oxygen, and that imparied in vivo activity may possibly be mediated by effects other than irreversible metabolic damage to these cells. The mechanism for the observed stimulation of phagocytosis remains to be determined.
Full text
PDF

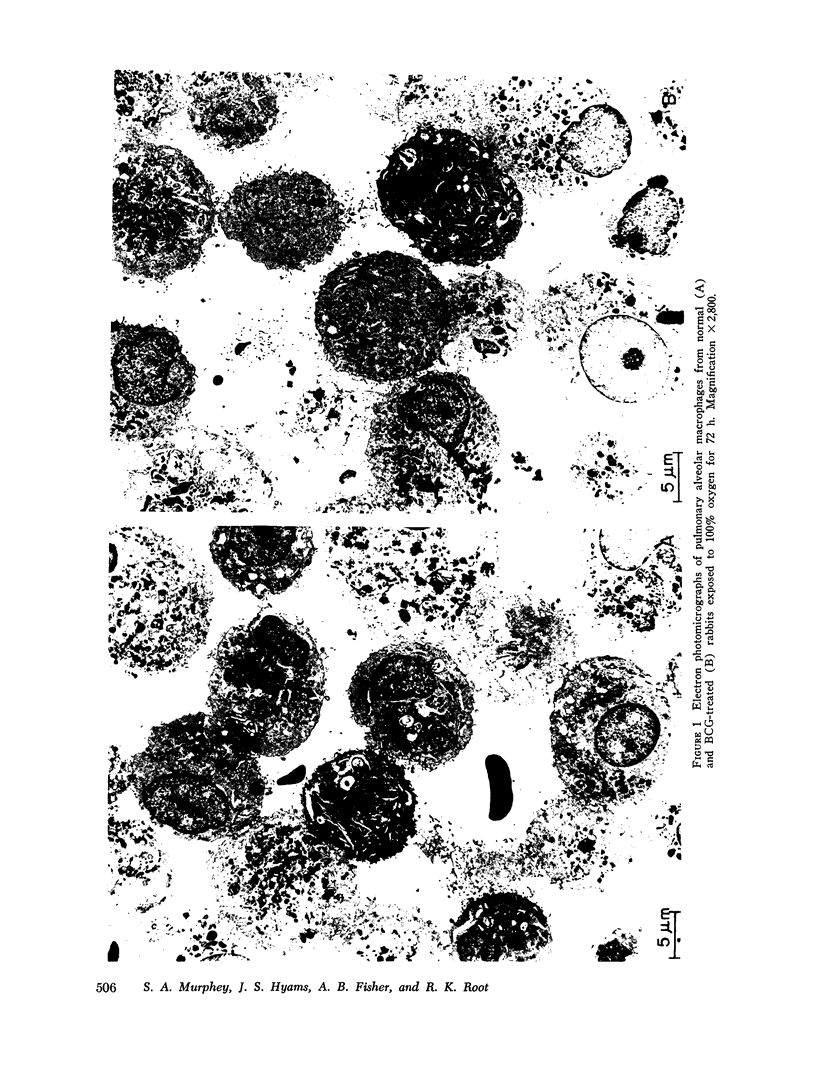


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Fisher A. B., Diamond S., Mellen S. Effect of O2 exposure on metabolism of the rabbit alveolar macrophage. J Appl Physiol. 1974 Sep;37(3):341–345. doi: 10.1152/jappl.1974.37.3.341. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. B., Vassallo C. L., Bell P., Kaskin J., Basford R. E., Field J. B. Catalase-dependent peroxidative metabolism in the alveolar macrophage during phagocytosis. J Clin Invest. 1970 Jun;49(6):1280–1287. doi: 10.1172/JCI106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. The J. Burns Amberson Lecture--in defense of the lung. Am Rev Respir Dis. 1970 Nov;102(5):691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- HEISE E. R., MYRVIK Q. N., LEAKE E. S. EFFECT OF BACILLUS CALMETTE-GU'ERIN ON THE LEVELS OF ACID PHOSPHATASE, LYSOZYME AND CATHEPSIN IN RABBIT ALVEOLAR MACROPHAGES. J Immunol. 1965 Jul;95:125–130. [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Good R. A. Fatal granulomatous disease of childhood. An inborn abnormality of phagocytic function. Lancet. 1966 Jun 4;1(7449):1225–1228. doi: 10.1016/s0140-6736(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Kass E. H., Green G. M., Goldstein E. Mechanisms of antibacterial action in the respiratory system. Bacteriol Rev. 1966 Sep;30(3):488–497. doi: 10.1128/br.30.3.488-497.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- MILLICAN R. C., RUST J. D. Efficacy of rabbit pseudomonas antiserum in experimental Pseudomonas aeruginosa infection. J Infect Dis. 1960 Nov-Dec;107:389–394. doi: 10.1093/infdis/107.3.389. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Massaro D. Alveolar cells: incorporation of carbohydrate into protein and evidence for intracellular protein transport. J Clin Invest. 1968 Feb;47(2):366–374. doi: 10.1172/JCI105733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi E., Selvaraj R. J., Sbarra A. J. The biochemical activities of rabbit alveolar macrophages during phagocytosis. Exp Cell Res. 1965 Dec;40(3):456–468. doi: 10.1016/0014-4827(65)90226-0. [DOI] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin P. A., Permutt S., Riley R. L., Radford E. P. Pulmonary antibacterial defenses with pure oxygen breathing. Proc Soc Exp Biol Med. 1971 Sep;137(4):1202–1208. doi: 10.3181/00379727-137-35756. [DOI] [PubMed] [Google Scholar]
- Tan J. S., Watanakunakorn C., Phair J. P. A modified assay of neutrophil function: use of lysostaphin to differentiate defective phagocytosis from impaired intracellular killing. J Lab Clin Med. 1971 Aug;78(2):316–322. [PubMed] [Google Scholar]
- Winter P. M., Smith G. The toxicity of oxygen. Anesthesiology. 1972 Aug;37(2):210–241. doi: 10.1097/00000542-197208000-00010. [DOI] [PubMed] [Google Scholar]