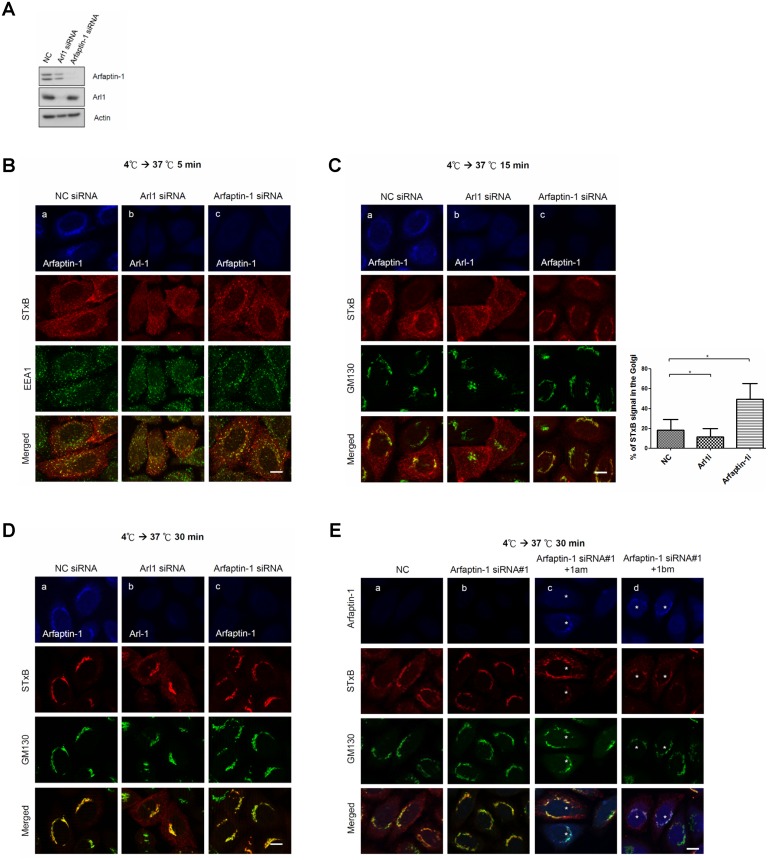
Fig 1. Arfaptin-1 regulates STxB transport from endosomes to the Golgi apparatus.
(A) HeLa cells were transfected with control siRNA or siRNA specific for Arl1 or arfaptin-1, as indicated. After 48 h, the cells were subjected to western blotting with anti-arfaptin-1 and anti-Arl1 antibodies, respectively. Actin was used as the internal control. Simultaneously, the knockdown cells were incubated with Cy3-conjugated STxB at 4°C for 20 min and shifted to 37°C for 5 min (B), 15 min (C) and 30 min (D), followed by immunofluorescence staining with anti-EEA1, anti-GM130, anti-Arl1 and anti-arfaptin-1 antibodies as indicated. The intensity and area of STxB (red) and GM130 (green) signals were quantified as described in the Materials and Methods. The percentage of STxB signal in the Golgi was calculated using the following formula: % of STxB signal in the Golgi = total intensity of co-localization of STxB and GM130/total intensity of STxB. The results are presented as the means±SDs; p<0.05 indicates significance, as assessed by one-way ANOVA. (E) Exogenous expression of siRNA-resistant arfaptin-1 in arfaptin-1-knockdown cells rescued the kinetics of STxB transport. HeLa cells were transfected with control siRNA or siRNA specific for arfaptin-1. After 24 h, the cells were transfected with siRNA-resistant arfaptin-1 (arfaptin-1am-myc or arfaptin-1bm-myc) for an additional 24 h, and a STxB transport assay with incubation at 37°C for 30 min was conducted. The cells were fixed and stained with anti-GM130 and anti-myc antibodies as indicated. Scale bars, 10 μm. Asterisks indicate siRNA-resistant arfaptin-1-expressing cells.