Fig 4. ELISA to characterize VLP surface-display of RG1-peptide and to detect antibodies to RG1 induced by 18L1–45RG1 VLP vaccination.
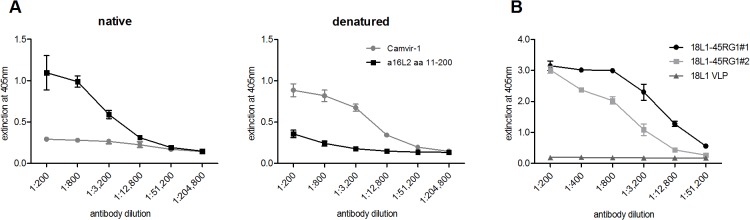
(A) Native or denatured HPV18L1–45RG1 VLP were attached to 96-well ELISA plate and contacted with serial dilutions of anti-HPV16 L2 polyclonal serum recognizing the RG1 epitope. The anti-L2 serum recognized both native and denatured chimeric VLP, indicating RG1 display on the surface of VLP. As a control, binding of mAb Camvir-1 was assessed, which is directed to a linear L1 epitope hidden in the assembled protein. (B) Biotinylated peptides representing HPV45 RG1 were attached to 96-well Streptavidin plates. ELISA was performed in triplicates using either immune sera raised to HPV18L1–45RG1 VLP or HPV18L1 VLP, or pre-immune sera, with dilutions ranging from 1:200 to 1:51,200. HPV18L1–45RG1 immune sera, but not HPV18L1 VLP sera or pre-immune sera, bound to the RG1 peptide at titers of 12,800 to 51,200 (serum #2 and #1, respectively). Data are shown as mean OD ± standard deviation (SD) and titers reported as mean values 3 SD above background signals (pre-immune values).
