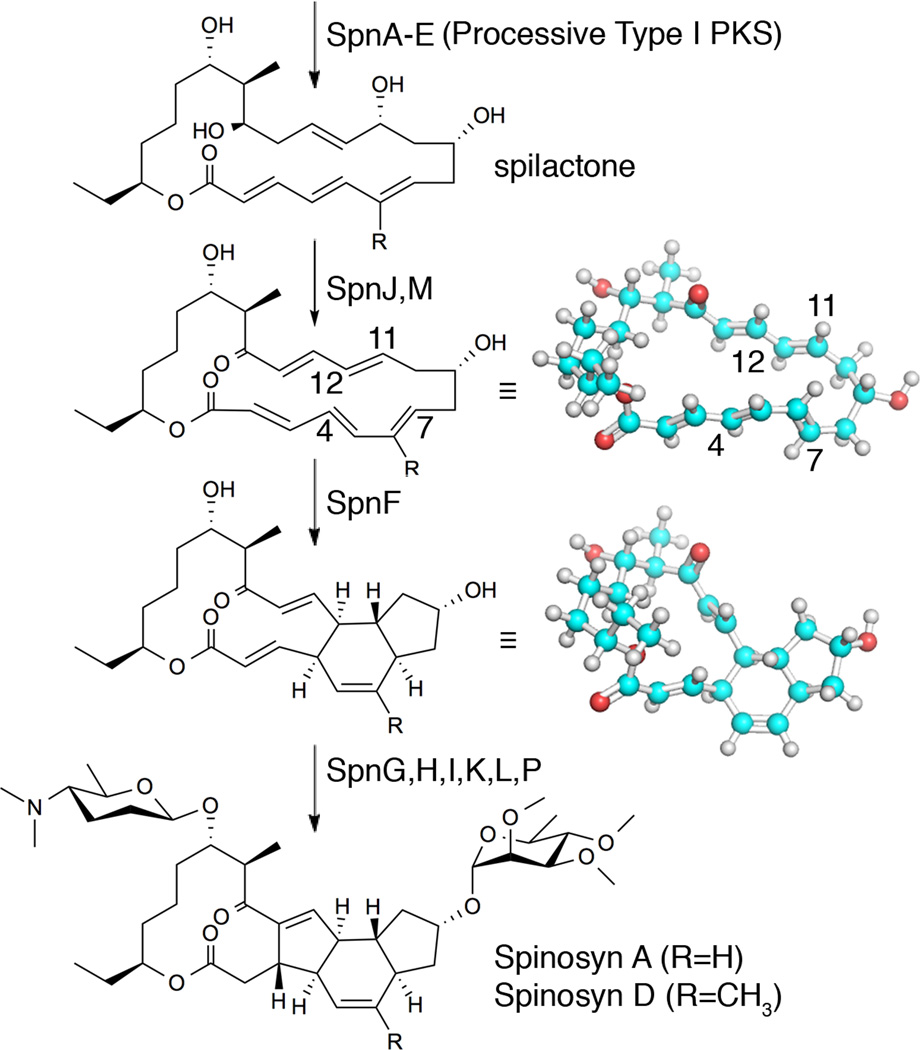
Figure 1.
Reactions mediated by SpnF and accompanying tailoring enzymes. The processive spinosyn Type I PKS generates spilactone, which is processed by several tailoring enzymes into spinosad (Spinosyns A and D). SpnF performs a [4+2]-cyclization, possibly through a Diels-Alder mechanism, between the C4–C7 diene (C5–C6 bond in s-cis geometry) and the C11–C12 dienophile, embedding a cyclohexene ring in the product (conformations minimized with the MMFF94s force field).