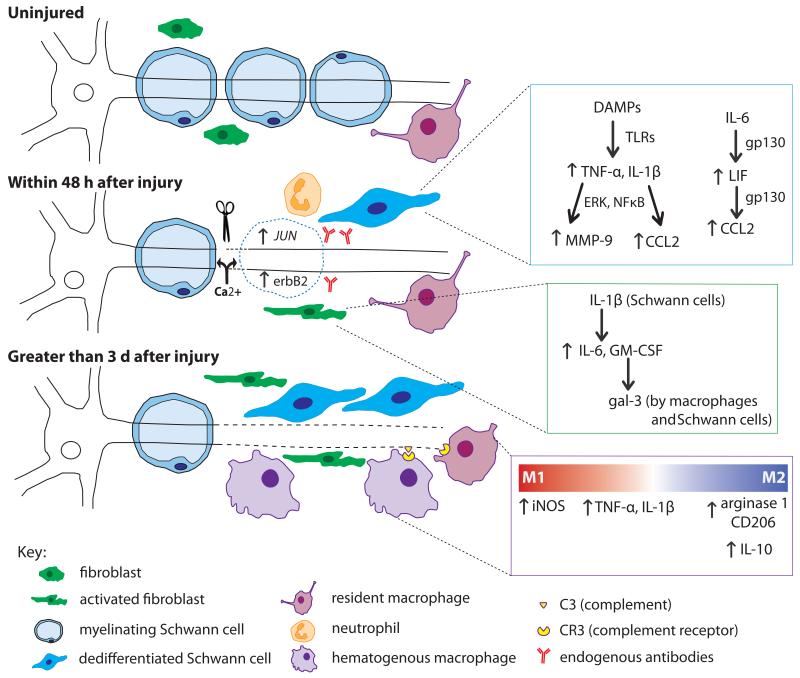
Figure 3.
The cells and molecules involved in WD in peripheral nerve. Within minutes of axonal injury, Ca2+ enters the proximal and distal nerve and initiates axonal breakdown. The resulting DAMPs stimulate the dedifferentiation of Schwann cells through the upregulation of JUN and erbB2. Through TLR signaling, dedifferentiated Schwann cells upregulate proinflammatory molecules (blue inset). Increased TNF-α and IL-1β activity leads to upregulation of both MMP-9 and CCL2. Schwann cells also respond to IL-6 from fibroblasts, which through gp130 signaling, triggers an autocrine cascade of LIF and CCL2 upregulation. Fibroblasts respond within hours by upregulating IL-6 and GM-CSF, the latter of which leads to gal-3 expression by macrophages and Schwann cells (green inset). Neutrophils infiltrate the injury site briefly within the first day after injury. Endogenous antibodies respond to nerve injury by activating complement. Three days post injury, macrophages have begun to infiltrate the nerve. Activated macrophages seem to play a dual role in WD evident by the factors they secrete (purple inset). Initially, they contribute to the inflammatory state by their production of TNF-α and IL-1β. CR3 on macrophages binds complement on degenerating myelin inducing phagocytosis. There is controversy over the subtype of macrophage involved; M1 markers have been shown at 1 and 3 d after injury, yet M2 markers have been found as early as 3 d and remain elevated for at least 14 d. It is thought that M2 macrophages are involved in regulating the inflammatory response in WD by upregulating the anti-inflammatory cytokine IL-10 leading to downregulation of pro-inflammatory cytokines once myelin degradation is complete.