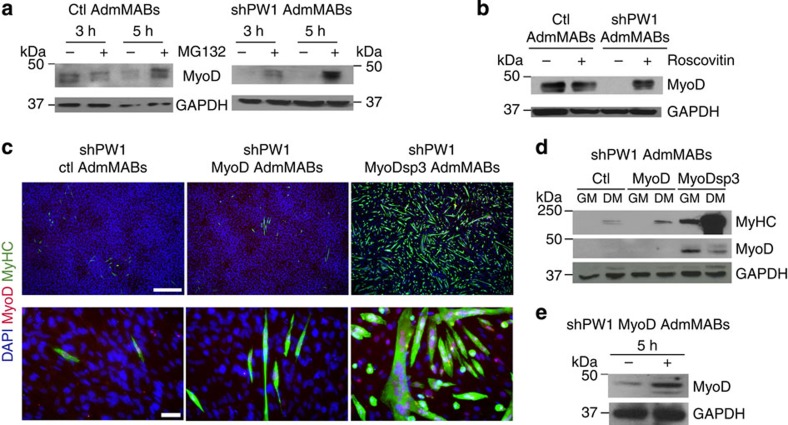
Figure 3. Silencing of PW1 leads to MyoD degradation via cycE/Cdk2-proteasome-dependent pathway.
(a) Western blot analysis of MyoD accumulation in Ctl and shPW1 AdmMABs following treatments with 50 μM of the proteasome inhibitor MG132, for 3 and 5 h and (b) 5 μM of the cdk2 inhibitor, Roscovitin for 5 h.The +refers to MG132- or Roscovitin-treated cells, whereas − refers to only DMSO-treated cells. GAPDH was used to normalize the amount of loaded proteins. (c) Immunofluorescence staining for myosin heavy chain (MyHC, green), MyoD (red) and nuclei (DAPI) on shPW1 AdmMABs transduced with retroviral vector expressing wt MyoD (shPW1 MyoD AdmMABs), mutated MyoD (shPW1 MyoDsp3 AdmMABs) and empty control vector (shPW1 Ctl AdmMABs). Scale bars, 500 and 75 μm. (d) Western blot analysis of the experiment described in c: MyoD and myosin heavy chain (MyHC) expression were checked in proliferating (GM) and differentiated (DM) shPW1 AdmMABs transduced with retrovirus expressing wt MyoD (MyoD), mutated MyoD (MyoDsp3) and empty control vector (Ctl). GAPDH was used to normalize the amount of loaded proteins. (e) MyoD expression, evaluated by western blot, in shPW1 AdmMABs stably transduced with the retrovirus expressing the wt MyoD (shPW1 MyoD) and treated for 5 h with the proteasome inhibitor MG132. The +refers to MG132-treated cells, whereas − refers to only DMSO-treated cells. GAPDH was used to normalize the amount of loaded proteins.