Figure 1.
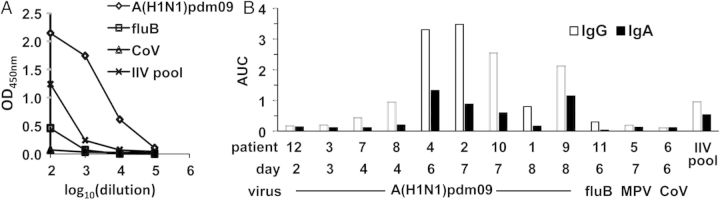
2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) binding activity of plasmablast-derived polyclonal antibodies (PPAbs). PPAbs from patients with acute influenza-like illness (ILI) were prepared from blood samples collected 2–8 days after disease onset. A pool of PPAbs from a subset (n = 23) of 2010/2011 inactivated influenza vaccine (IIV) recipients (IIV pool) was also tested. This PPAb pool was assembled by combining equal amounts of immunoglobulin G (IgG) from each PPAb sample prepared with blood samples collected 6–8 days after IIV receipt. A, IgG enzyme-linked immunosorbent assay (ELISA) titration curves of 4 serially diluted PPAb samples: representative ILI patients 4 (A[H1N1]pdm09), 11 (influenza B virus [fluB]), and 6 (coronavirus [CoV]) and the PPAb pool from IIV recipients (IIV pool). B, IgG and immunoglobulin A (IgA) binding activity, shown as area under curve (AUC) of ELISA titration curves of individual ILI PPAb samples and the IIV PPAb pool. Patient number, days between disease onset and blood collection, and infecting agent are shown below the relevant bars in the graph.
