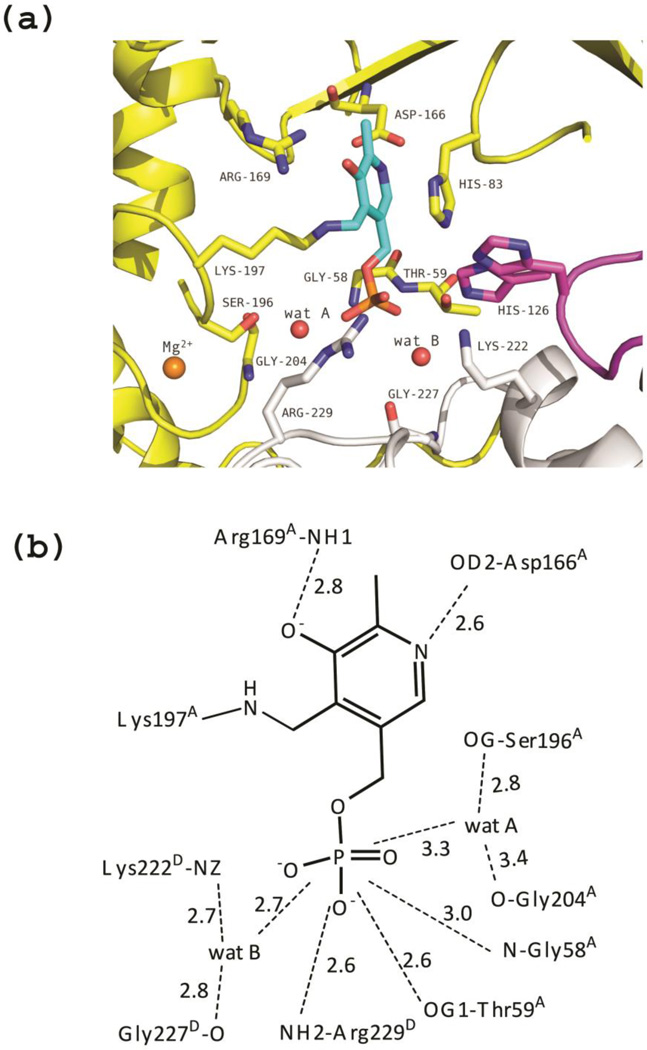
Figure 4.
Unliganded eTA. (a) View of the active site of monomer A, showing PLP (in cyan) bound as internal aldimine to Lys197. Three monomers (A, B and D colored as in Fig. 3) contribute to form the active site. Residues of loop 2 from monomer D (222–230) are shown as grey sticks. Loop 3 contributed by monomer B (residues 121–131) is shown in magenta; two different conformations of H126B are shown. (b) Scheme of two-dimensional contacts among PLP, protein residues and the structural water molecules discussed in the text. Dashed lines indicate hydrogen-bond interactions and their length (in Å).