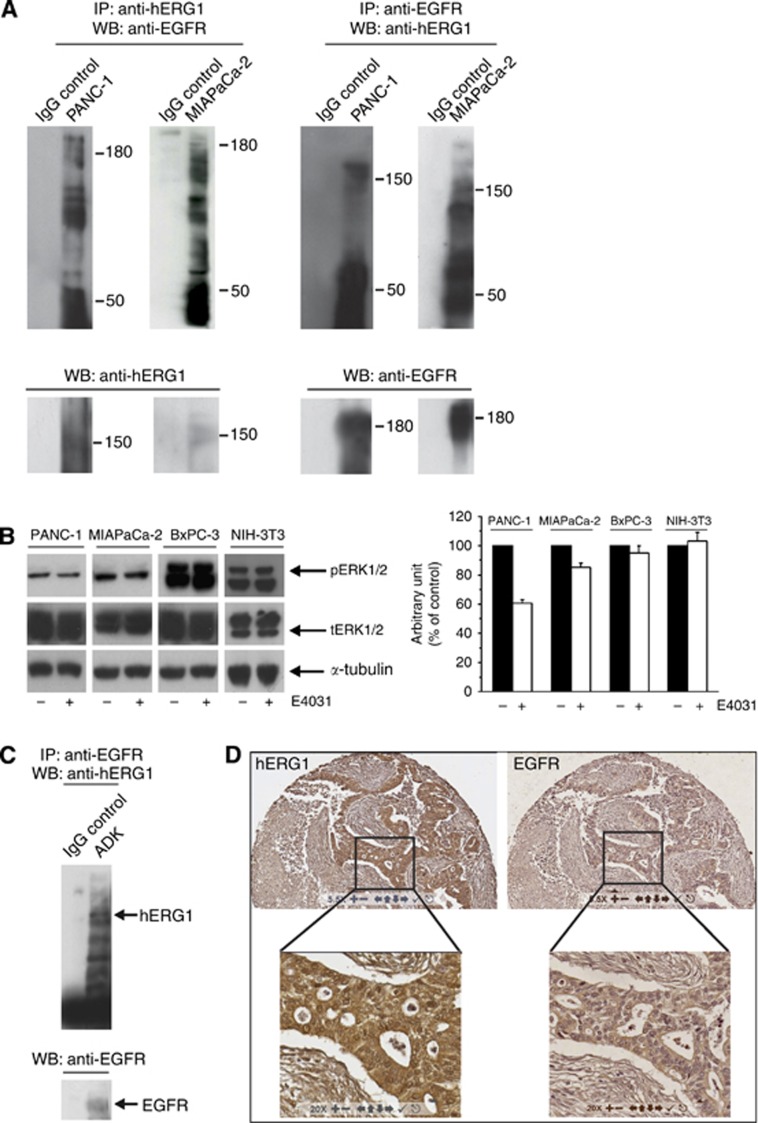
Figure 3.
Relationships between hERG1 and EGF-R in PDAC cell lines and primary samples. (A) Co-immunoprecipitation (co-IP) of hERG1 and EGF-R in PANC-1 and MIAPaCa-2 cells (expressing EGF-R at high levels (Supplementary Information, Supplementary Figure S1). Co-IP experiments were performed using either a α-hERG1-MoAb (panels on the right) or the anti-EGF-R (sc-03, Santa Cruz, Dallas, TX, USA; panels on the left) to immunoprecipitate and the same EGF-R antibody or the polyclonal antibody directed against the hERG1 C-terminus (C54 antibody) for membrane decoration. (B) Effect of E4031 on ERK1/2 phosphorylation after 3 h treatment. NIH-3T3 cells were used as negative control. For membrane decoration, phospho-p44/42 MAP kinase (Cell Signaling, Danvers, MA, USA) and ERK1 antibodies (C-16, Santa Cruz), that are able to recognize both ERK1 and ERK2 total protein were used. Monoclonal anti-α-Tubulin antibody (Sigma-Aldrich) was used as a probe for α-Tubulin as an internal loading control. ***P<0.01 (Student's t-test). (C) Co-IP of hERG1 and EGF-R in PDAC primary samples. Co-IP experiments were performed on a total protein extract (1 mg) pooled from three different primary PDAC samples, using the same protocol as in panel A. (D) IHC for hERG1 and EGF-R. A representative picture of IHC staining with the two antibodies in the same tumour sample is shown. Magnification: 55x (upper panels) and 200x (lower panels).