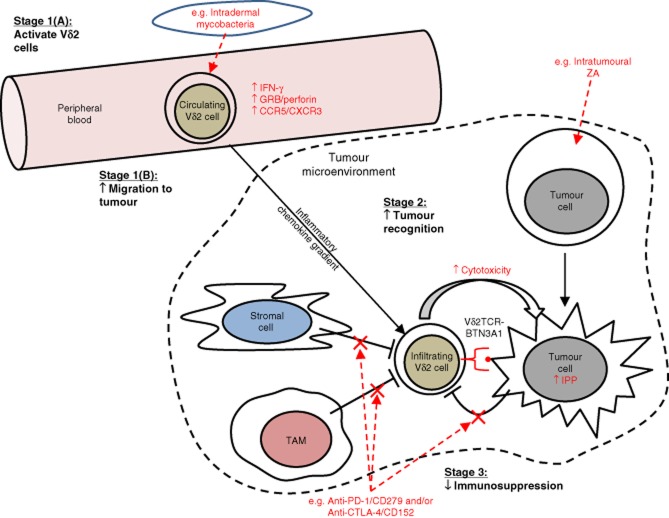
Figure 1.
Schematic overview of potential strategies to harness the power of Vδ2 cells in cancer immunotherapy. In this schematic, we have depicted and compartmentalized into key stages the hypothetical processes involved in achieving optimal Vδ2 cell targeting of tumour and the potential ways in which clinical intervention may be used to manipulate the activity of Vδ2 cells in patients. ‘Stage 1(A): activate Vδ2 cells': peripheral blood Vδ2 cells must be primed in order to enhance their anti-tumour response. Intradermal administration of mycobacterial preparations such as bacillus Calmette–Guérin (BCG) may achieve this by increasing Vδ2 cell expression of cytolytic effector molecules [e.g. granzyme B (GRB) and perforin] and production of proinflammatory cytokines [e.g. interferon (IFN)-γ]. ‘Stage 1(B): increase migration to tumour’: primed Vδ2 cells must then migrate from the peripheral blood to the site of tumour. This may involve inflammatory chemokines, and thus require optimal expression of inflammatory chemokine receptors on Vδ2 cells following the priming stage. ‘Stage 2: increase tumour recognition’: Once Vδ2 cells have successfully infiltrated the tumour mass, tumour cells must be susceptible to Vδ2 cell cytotoxicity. Vδ2 cell recognition of tumour can be manipulated in certain cancers by delivering nitrogen-containing bisphosphonates (NBPs) such as zoledronic acid (ZA) to the site of the tumour. ‘Stage 3: decrease immunosuppression’: immunosuppressive elements within the tumour microenvironment must be counteracted in order to ensure an uninhibited Vδ2 cell response against tumour. Examples of potential immunosuppressive pathways include programmed death (PD)-1/CD279 and cytotoxic T lymphocyte antigen-4 (CTLA-4)/CD152, both of which can be blocked using monoclonal antibodies.