Abstract
Forkhead box protein 3 (FoxP3)+ regulatory T cells (Tregs) are important not only in regulating the development of autoimmune conditions, but also in chronic infectious diseases. Given their cardinal function in suppressing immune activation, research has focused upon whether they play a detrimental role in chronic infections, particularly HIV. While the role of Tregs in HIV has been investigated intensively, it remains an unresolved topic. However, it is generally accepted that Tregs are susceptible to HIV infection and are preferentially preserved over conventional CD4+ T cells. It is unknown whether the peripheral-induced or the thymic-derived Tregs are more susceptible to HIV cytotoxicity. It has been recognized that Tregs can be segregated into two subsets based on Helios expression, with the vast majority being Helios+. This study examines the impact of HIV infection on total Tregs and their Helios subsets in a perinatal-acquired HIV-infected paediatric population. The finding indicates a selective expansion or survival of Tregs in association with CD4 depletion and increased viraemia. The Helios+ and Helios− subsets within Tregs appear to be equally affected. However, the Helios+ Tregs seem to be more preserved in patients with low CD4+ ≤ 25% and detectable plasma HIV RNA >20 copies/ml. In this group, the frequencies of Tregs are increased, but their numbers appear insufficient to restrain immune activation. In conclusion, our findings suggest that both Helios subsets of Tregs are susceptible to HIV infection and are preferentially preserved compared to conventional CD4+ T cells.
Keywords: AIDS, regulatory T cells, T cells
Introduction
Since the demonstration that depletion of CD4+CD25+ T cells results in the development of organ-specific and systemic autoimmune disease, accumulating evidence increasingly supports the involvement of forkhead box protein 3 (FoxP3)+ regulatory T cells (Tregs) in diverse immune disorders, cancers and infectious diseases 1–3. Tregs are thought to be involved in modulating the responses of the immune system, thereby affecting the outcome of many chronic viral infections such as herpes simplex virus (HSV), hepatitis C virus (HCV) and retroviruses, including HIV 4. In HIV, demonstration of a direct and predictable relationship of Tregs with the disease process in acute or chronic infection or subsequent immune dysregulation (as evidenced by increased activation markers) has been extremely challenging 5. It remains unclear whether Tregs are beneficial to the host by regulating chronic immune inflammation or detrimental by suppressing anti-HIV immunity 6,7.
Studies in adults show that CD4+CD25+ Tregs and in-vitro FoxP3 transduced conventional CD4+ T cells are susceptible to HIV infection 8,9. In addition to CD4 and CD25, they can express the chemokine co-receptor CCR5, a required co-receptor for HIV entry into cells 8. CXCR4 co-receptor is expressed, but at lower levels compared to CCR5. The use of replication-competent HIV demonstrates that HIV replicates efficiently in Tregs and is cytotoxic to the cells. While some studies report that Tregs may be preferentially infected and depleted 10, one study showed variable susceptibility of Tregs to HIV depending on trophism, virus strain and viral life-cycle timing 9. However, the Tregs remained suppressive 24 h after infection in vitro.
Tregs may suppress immune activation, as demonstrated by enhanced in-vitro HIV-specific activity, cytokine production and proliferative responses of T cells 11,12. Therefore, Tregs may have a protective role in the pathogenesis of HIV by limiting the dysregulated immune activation seen in HIV that precedes the collapse of the immune system. In contrast, Tregs may suppress effective anti-viral responses to HIV infection by targeting HIV-specific effectors. These seemingly dichotomous and antagonistic roles of Tregs are difficult to delineate clearly 13,14. On one hand, Tregs may facilitate the establishment of HIV by inhibiting HIV-specific immunity. On the other hand, Tregs may modulate the non-specific inflammation that is detrimental. Still others propose that the perturbation of Tregs in HIV is not the direct cause of immune activation noted in HIV infection and that the data do not show Tregs as playing a significant role in temporizing the immune response to HIV 15.
There are conflicting data in the literature regarding the role of Tregs in HIV infection and their subsequent interaction. Some studies in adults demonstrated the proportion (%) of Tregs (defined as CD4+CD25+FoxP3+) to be lower in viraemic patients, with a concomitant increase in activation markers, human leucocyte antigen D-related (HLA-DR) and CD38 on CD8 16. Similarly, another study showed a gradual decrease of the absolute and proportion of Tregs (defined as CD3+CD4+CD25hiFoxP3+) during HIV disease progression, together with increased immune activation 17. In one study of patients with acute primary HIV infection (median 13 days), the frequency of Tregs was found to be lower than in chronic patients and, over time, the frequency of Tregs decreased in untreated patients 18. In addition, the elevated proportion of Tregs and low levels of immune activation, evidenced by reduced expression of the activation marker CD69 in a cohort of HIV-resistant sex workers exposed to HIV regularly who remained negative, was reported in another study 19. Alternatively, studies showed that in HIV patients with low CD4 counts (<200), absolute Tregs (defined as CD4+FoxP3+) were lower but constituted a higher proportion of the CD4 population compared to HIV-positive patients with higher CD4 counts and healthy adults 20.
Very few studies have investigated Tregs in HIV-infected paediatric patients 21,22. In one such study, the frequency of Tregs correlated positively with viraemia but negatively with CD4 cells, suggestive of Treg expansion with CD4 decline 21. Tregs declined at a slower rate than other CD4 cells. There is a selective expansion of Tregs associated with viraemia and CD4 depletion. In another study of Tregs in HIV-exposed but uninfected neonates, unexposed neonates and infected neonates, high levels of Tregs and low levels of CD4+ and CD8+ T cell activation were documented in the exposed uninfected neonatal cord blood 22. Unlike in the unexposed neonates, exposed neonates had an HIV-1-specific T cell response. The depletion of CD4+CD25+CD127− Tregs augmented this HIV-specific response, suggesting that Tregs may contribute to protection from vertical transmission by suppressing T cell activation, which is important for virus uptake into cells.
It is recognized that Tregs exist as two subsets, thymic-derived (tTregs) and peripheral-induced (pTregs), with many similarities and differences 23. The challenge has been the difficulty in distinguishing these two subsets in vivo in order to understand their roles in different diseases. The transcription factor Helios is suggested as a potential biomarker expressed preferentially in tTregs 24. However, subsequent studies have challenged this claim, resulting in a controversial and unresolved topic 25. In HIV, there is no study that investigates whether the Helios+ and Helios− Treg subsets are affected differentially during the different stages of infection. Given the profound level of CD4 depletion, regeneration and activation depending on the HIV infectious status of the patients, we investigated the impact of CD4 depletion and HIV viraemia on Tregs and their Helios+ subset in our perinatally acquired HIV-infected paediatric cohort. We found that a selective expansion or survival of Tregs associated with CD4 depletion and increased viraemia. Interestingly, both Helios+ and Helios− subsets within Tregs appeared to be equally affected. However, when segregating our patients into three groups based on CD4% and HIV RNA, the poor status group with low CD4+ ≤ 25% and detectable plasma HIV RNA >20 copies/ml had increased frequencies of Tregs and preservation of Helios+ Tregs. Nevertheless, this group also had the highest immune activation based on increased CD8+ T cells and their up-regulation of CD38 and HLA-DR expression. While there was a selective preservation of Tregs and the Helios+ subset, it remains unclear whether they played a positive or negative role in paediatric HIV disease and progression.
Materials and methods
Patients
All perinatally HIV-infected patients attending the Pediatric HIV Clinic of UTHealth (Houston, Texas, USA) and providing informed consent were eligible for inclusion into this prospective cross-sectional study. Sixty patients meeting these criteria provided blood samples during the study interval; this group represented 87% of the clinic's perinatally infected patients. No perinatal transmissions were from breastfeeding. The fraction of intra-utero and intrapartum transmissions was not available. The study was approved by the Institutional Review Board of UTHealth. Clinical and related laboratory data were from medical records. The percentages and numbers for CD3, CD4, CD8, CD38 and HLA-DR were obtained commercially through Labcorp.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from ethylenediamine tetraacetic acid (EDTA) anti-coagulated fresh whole blood by Ficoll gradient centrifugation. A total of 50 000 cells were surface-stained with anti-CD4 (RPA-T4; Biolegend, San Diego, CA, USA) and intracellularly stained with anti-Helios (22F6; BioLegend) and anti-FoxP3 (259D; BioLegend) using the FoxP3/transcription factor staining buffer set, as per the manufacturer's protocol (eBioscience, San Diego, CA, USA). The fluorescence activated cell sorter (FACS)Calibur (BD Biosciences, San Jose, CA, USA) was used for data acquisition. Data analysis employed FlowJo version 7·6 (Tree Star, Inc., Ashland, OR, USA).
Analysis
Because previous studies 6,21 have demonstrated that Tregs may associate with clinical measures of HIV disease, patients were classified into cohorts based on clinical laboratory indicators of HIV disease status, where good status was defined as CD4+ ≥ 25% (CD4↑) and undetectable plasma HIV RNA <20 copies/ml [viral load (VL)↓]. Of the four resulting cohorts (CD4↑VL↓, CD4↓VL↑, CD4↑VL↑, CD4↓VL↓), the cohort with CD4↓VL↓ was excluded because it comprised only one patient. The remaining cohorts were referenced as HIV clinical status good (CD4↑VL↓), intermediate (CD4↑VL↑) or poor (CD4↓VL↑).
Clinical laboratory findings were reviewed to characterize patients' status near the time of Treg measurements (± 7 days). Indicators of the severity of HIV disease during the course of infection included Centers for Disease Control (CDC) immunological and clinical status (which provided an estimate of a patient's most severe immune suppression and worst clinical status), nadir CD4% and nadir number of CD4+ cells/μl. Anti-retroviral drug treatment (ART) status at the time of Treg measurement was classified as (1) combined ART (cART), which most often comprises dual-nucleoside/nucleotide reverse transcriptase inhibitors with either a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor; (2) ART, for those who did not meet the inclusions for cART; or (3) no ART regimen. Patient history of exposure to ART comprised classifications cART, ART and no ART during the course of infection. An individual patient may be classified into one or more than one of these groupings. Cumulative anti-retroviral drug major resistance mutations (mRM) was the sum of a cumulative list of unique mRM found in each patient's records from the first RM test to the day of the Treg blood sample that are clinically significant, and resulted in resistance to the ART in use. mRM were those defined in the Stanford database (http://hivdb.stanford.edu/; date accessed 15 July 2013).
Measured outcomes (Tregs and Helios+ subset) and clinical results were compared between the three HIV disease status cohorts by Kruskal–Wallis test with pairwise comparisons. Main-effects generalized linear models with either identity or log link was used to test the association of Treg outcomes (both as percentage and cell number for each outcome) with the three clinical cohorts while controlling for age, gender, ethnicity, CDC clinical categories and CDC immunological categories. Residuals for all models were checked for normality by Shapiro–Wilks test to ensure the appropriate fit of the model to the outcome measure.
Descriptive data are reported as median with 25th and 75th percentiles and minimum and maximum values for continuous variables and counts (percentage) for categorical variables. Comparisons between groups were by non-parametric tests, as the data deviated from a normal distribution as assessed by Q–Q plots and Shapiro–Wilks test. The Mann–Whitney U-test was used for comparisons between two groups and the Kruskal–Wallis test was used for comparing more than two independent groups. Box-and-whiskers plots represented 10th and 90th percentiles; points outside whiskers were outliers. Correlations were calculated by Spearman's rho test. Data management and analysis employed Microsoft Access (Microsoft Inc., Redmond, WA, USA) and spss version 19 (Statistical Package for Social Sciences, Chicago, IL, USA), respectively. As the majority of outcomes were co-linear, multivariate analyses were limited.
Results
Patient population and selected characteristics
Sixty patients were included in the study. The population attended the UTHealth clinic for a median of 14·4 years and at the time of Treg determinations were comprised mainly of male patients (48·3% female) on cART (83·3%), who were black (70%), teenagers (median, 14·4 years old), with good immunological status (32% CD4) and with low HIV viraemia (65 HIV RNA copies/ml). The population has had more severe HIV disease in the past with median 18% CD4 nadir (Table 1).
Table 1.
Patient characteristics and outcomes
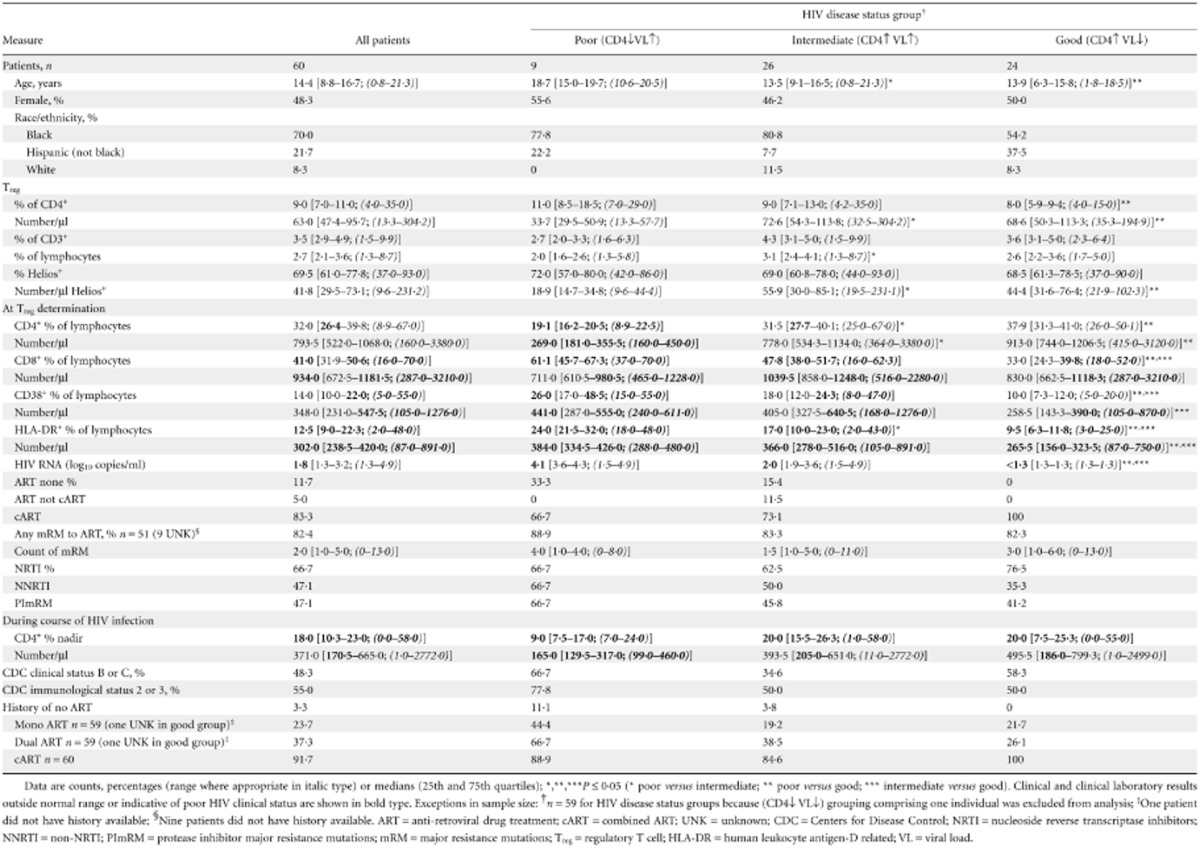
A median of 9% CD4+ cells were Tregs based on FoxP3 expression and 69·5% of the Tregs were Helios+. Notable abnormal clinical values at the time of Treg measurements included increased CD8+ cells and increased activated CD8+ cells based on their expression of HLA-DR. The majority has mRM to ART drugs (82·4%) and were on cART at some point in their disease course (91·7%).
To further resolve the relationship between HIV disease status and Treg findings, outcomes were parsed into cohorts comprising good (CD4↑VL↓), intermediate (CD4↑VL↑) and poor (CD4↓VL↑) disease status (Table 1), based on their immunological and virological status at the time of collection of the blood sample for Treg measurements. There were significant differences in age and race/ethnicity distribution among the HIV disease status groups, with older patients being significantly more frequent in the poor disease status group (median age 18·7, 13·5 and 13·9 for the poor, intermediate and good groups, respectively, P ≤ 0·05) and white patients being significantly less likely to be in the good clinical status group compared to the intermediate clinical status group (8·3 versus 11·5%, P ≤ 0·05). Not surprisingly, there were more patients in poorer CDC immunological status in the poor disease status group than in the good disease status group (77·8 versus 50%, P ≤ 0·05), but this disparity was not seen for the CDC clinical status classification. There were more patients in the poor disease status group who were never on ART compared to the good disease status group (33·3 versus 0%, P ≤ 0·05) (Table 1).
As expected, VL was correlated negatively with percentage and absolute CD4 counts (r = −0·514, r = −0·420, P < 0·001), consistent with depletion of CD4+ T cells with viraemia. There was significant positive correlation between VL and percentages of CD8+ T cells as well as their expression of CD38 and HLA-DR (r = 0·613, r = 0·657, r = 0·646, P < 0·001), indicating immune activation with increasing viraemia.
Preservation of Tregs and their Helios+ subset in paediatric HIV
Figure 1 shows the gating strategy of two representative patients. Forward- and side-scatters were used to gate on lymphocytes (panel not shown) from which CD4+ cells were subsequently identified (Fig. 1a). The percentage of FoxP3+ Tregs was calculated within CD4+ (Fig. 1b). Figure 1c shows the correlation of FoxP3 and Helios within CD4+. The Helios+ Treg subset was determined based on the percentage of Helios within FoxP3+ Tregs (Fig. 1d).
Figure 1.
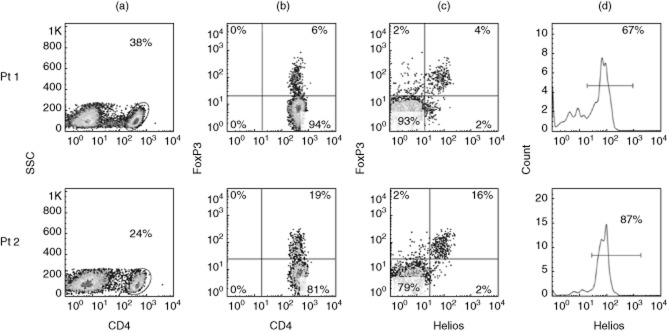
Gating strategy for regulatory T cells (Tregs) and Helios subsets. Representative fluorescence activated cell sorter (FACS) plots of two patients showing (a) the gating for CD4 within lymphocyte gate; (b) forkhead box protein 3 (FoxP3) expression within CD4; (c) correlation of FoxP3 and Helios within CD4; and (d) percentage of Helios within CD4+FoxP3+ Tregs.
To assess whether Tregs and their Helios+ subset were more preserved relative to conventional CD4+ T cells, we correlated their frequencies and absolute numbers with CD4 and viraemia. As CD4+ cells declined in frequencies and numbers, there was an increase in the frequencies of Tregs (r = −0·439 and r = −0·486, P < 0·001, respectively), while the Helios+ subset remained unchanged (Fig. 2a,b). The absolute numbers of Tregs and Helios+ correlated significantly and positively with absolute CD4 counts (r = 0·727 and r = 0·603, P < 0·001, respectively) (Fig. 2c). There was also a positive correlation for the frequencies (r = 0·291, P = 0·024) and numbers (r = 0·924, P < 0·001) of Tregs versus their Helios+ subsets (Fig. 2d). The direct positive correlation of the Helios+ subset with Tregs suggested that this Helios+ subset may be more preserved from thymic reconstitution or peripheral expansion as the frequencies of Tregs increase.
Figure 2.
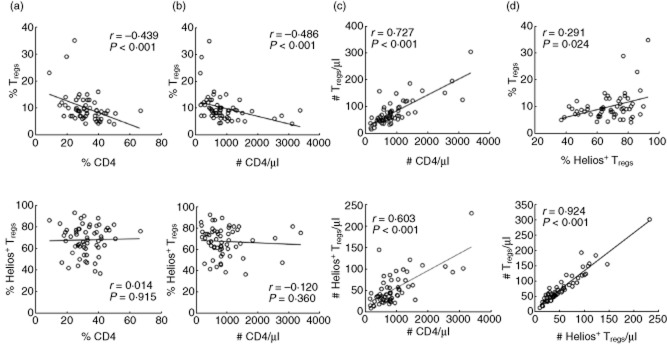
Preservation of regulatory T cells (Tregs) and their Helios+ subset in paediatric HIV. Correlation of the frequencies of Tregs and their Helios+ subset with (a) the frequencies and (b) numbers of CD4. (c) Numbers of Tregs and their Helios+ subset versus CD4 numbers. (d) Frequencies and numbers of Tregs versus Helios+ subset; n = 60.
Consistent with a selective expansion or preservation in Tregs, the frequencies of Tregs (r = 0·382, P = 0·003) and Helios+ subset (r = 0·101, P = 0·442) were not affected negatively by increased viraemia in contrast to CD4+ cells (r = −0·382, P = 0·003) (Fig. 3). Similarly, absolute numbers of Tregs (r = −0·286, P = 0·027) and Helios+ (r = −0·221, P = 0·09) were less affected by viraemia compared to CD4+ cells (r = −0·420, P < 0·001). These results suggested that Tregs and their Helios+ subset had relatively increased survival and/or expansion during viraemia and CD4 depletion.
Figure 3.
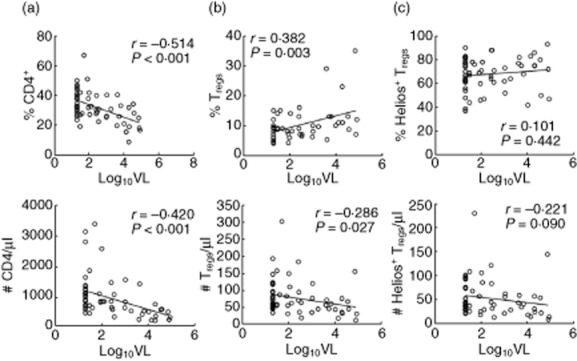
Preservation of regulatory T cells (Tregs) and Helios+ subset during viraemia. Correlation of percentages and numbers of (a) total CD4, (b) Tregs and (c) Helios+ subset with viral load; n = 60.
Effect of age on Tregs and their Helios+ subset
While the frequencies and numbers of CD4 declined with age, there was no significant change for the percentage of Tregs and the Helios+ subset (r = 0·168, P = 0·202 and r = −0·066, P = 0·617) (Fig. 4). However, the absolute numbers of Tregs and the Helios+ subset showed a significant negative correlation to age (r = −0·610, r = −0·571, P < 0·001, respectively), indicating a decline in their numbers with increasing age, even in those groups with good and intermediate clinical status (data not shown).
Figure 4.
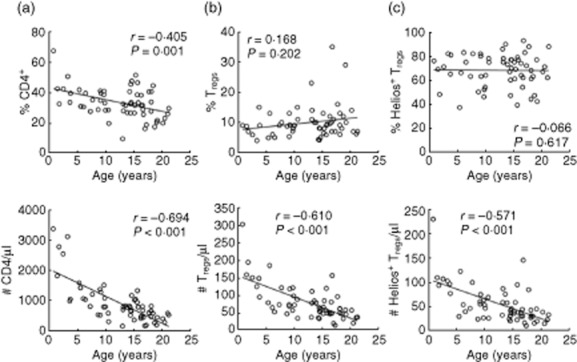
Percentages of regulatory T cells (Tregs) and Helios+ subset are unaffected by age. The frequencies and numbers of (a) total CD4, (b) Tregs and (c) Helios+ subset with age; n = 60.
Tregs were increased during immune activation
The proportion of Tregs was correlated significantly and positively with total CD8+ (r = 0·452, P < 0·001) and CD8+ subsets of immune activation markers, CD38 (r = 0·462, P < 0·001) and HLA-DR (r = 0·459, P < 0·001), suggesting the preservation of Tregs in the face of increasing immune activation and destruction with progressive disease (Fig. 5a–c). This relationship was also kept for absolute numbers of Tregs versus CD8+ and versus CD38+ (r = 0·495, r = 0·472. P < 0·001), but not HLA-DR+ (r = 0·037, P = 0·798). It is unclear whether the Tregs were responding or reacting to the immune activation and whether, in their absence, the immune response would be more fulminant.
Figure 5.
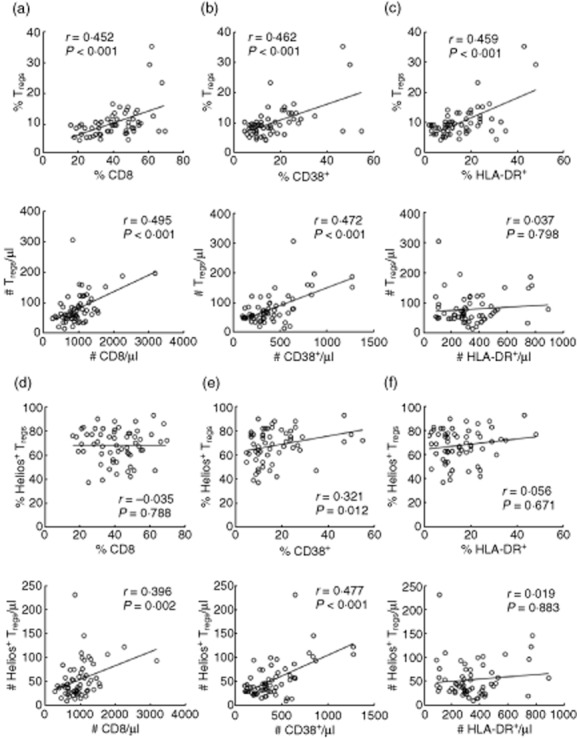
Increasing regulatory T cells (Tregs) with immune activation. The frequencies and numbers of Tregs (a–c) and Helios+ subset (d–f) versus total CD8, CD8+CD38+ and CD8+ human leucocyte antigen D-related (HLA-DR)+; n = 60.
While the proportion of Helios+ Tregs was correlated significantly and positively with CD38+ (r = 0·321, P = 0·012), there was no correlation with proportions of total CD8+ or HLA-DR+ (Fig. 5d–f). Absolute numbers of Helios+ Tregs were correlated significantly and positively with CD8 (r = 0·396, P = 0·002) and CD38 counts (r = 0·477, P < 0·001).
Selective preservation of Tregs and their Helios+ subset in the poor HIV category group
We next segregated our HIV cohort into three categories based on their CD4 and VL to examine the Tregs and their Helios+ subset. As expected, patients in the defined poor clinical status group had significantly increased percentages of CD8+ and CD8+CD38+ and both percentages and numbers of CD8+HLA-DR+ compared to patients in the good clinical status group (Table 1). These results support what is observed with immune dysregulation in poorly controlled or progressive HIV 26,27. When examining the ratio of Tregs and Helios+ subsets to their immune activation, the poor group had the lowest ratios to CD8, CD38 and HLA-DR, suggesting possible compromised immune regulation from the Tregs (Supporting information, Fig. S1).
Tregs in the poor clinical status group had significantly higher percentages than did the good group, but their numbers were lower (Fig. 6a). Non-parametric tests were used to compare groups in order to avoid the over-influence of outliers seen in the skewed data. The percentages of the Helios+ subset were similar among the three groups, although the absolute numbers were lowest in the poor group (Fig. 6b). In support of a preferential survival or expansion of Tregs and their Helios+ subset with increasing viraemia and CD4 depletion, there were higher Tregs and Helios+ subset : CD4 ratios in the poor group (Fig. 6c,d).
Figure 6.
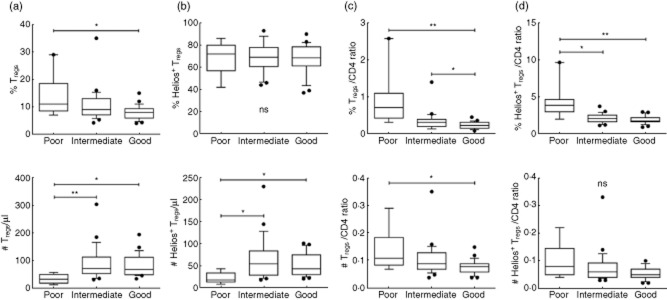
Preservation of regulatory T cells (Tregs) and Helios+ subset during CD4 lymphopenia and viraemia. The frequencies and numbers of (a) Tregs and (b) Helios+ subset; and (c) Treg/CD4 and (d) Helios+ Treg/CD4 ratios within the poor, intermediate and good HIV status groups. *P < 0·05; **P < 0·001.
Discussion
The outcome of this prospective cross-sectional study in a paediatric perinatal HIV-infected population revealed that while Tregs are susceptible to HIV-induced destruction based on reduced cell numbers, they have a higher preservation relative to CD4 decline associated with HIV viraemia. This selective conservation maintained a similar proportion of Helios+ and Helios− subsets within Tregs. However, within the poor group that had CD4 lymphopenia and high VL, there is evidence of greater preservation of the Helios+ Tregs within CD4+ T cells. The mechanisms responsible for changes in Treg numbers and precentages are not fully known, including the contribution of VL. The increased Tregs could be due to one or more of these possibilities: better survival, greater expansion, thymic reconstitution and/or de-novo generation. It is possible that the Helios+ Tregs were more susceptible to HIV-induced death, but their loss was offset by thymic reconstitution. Alternatively, this increased Treg proportion might have been contributed by generated pTregs that had up-regulated FoxP3, with some expressing Helios 28,29. It would be interesting to investigate whether this observation is similar or different in the adult HIV+ population, where thymic function is less preserved. The small paediatric blood volume and the lack of a reliable cell surface marker that would allow for selective isolation of Helios+ and Helios− subsets of Tregs limit the ability to examine their functions and Treg-specific demethylation region (TSDR) that has been noted to be specific to bona fide, stable Tregs 30. Moreover, it would be interesting to examine which Helios subsets might be more susceptible to HIV infection. Future longitudinal studies are needed to investigate Treg expansion, survival and turnover with regard to viraemia.
In our study, we focused only on the total population of Tregs within CD4+ T cells based on total FoxP3 expression and segregated the Tregs based on Helios expression. Nevertheless, our findings are similar to previous studies, with some using additional markers for their Treg quantification, such as CD25 and CD45RA 20,31–33. As Treg numbers decrease with CD4 depletion, their percentages increase within the CD4+. This observation is most evident in the chronically infected viraemic patients. When the patients are better controlled with good CD4 counts and undetectable HIV, their Treg percentages decrease to within the reported 5–10% in healthy donors 34. Even though our patient population was heterogeneous we were able to find correlations and differences, indicating the significance of our findings.
Although Tregs are critical for regulating immune homeostasis and activation it appears that, at a certain threshold, they lose control. While there was a selective increase in the proportion of Tregs during CD4 lymphopenia and HIV viraemia, there was also an increase in CD8+ expansion and activation based on up-regulation of CD38 and HLA-DR within CD8+ T cells. This increased turnover of Tregs correlated with hyperactivation and disease progression, as demonstrated by another study 35. The functions that Tregs and their Helios subsets were playing in this setting were unclear at this point. While their numbers were low, they might be selectively preserved to prevent or control the clinical manifestation of autoimmunity and inflammation. A recent study revealed that, in their HIV group with CD4 <15% that had low Treg numbers but increased frequencies, there was increased autoantibody production but no clinical autoimmune diseases 36. One possibility is that Tregs negotiate for the host survival by accepting HIV chronicity and progression. It is currently not possible to test whether, in their absence, the HIV disease course would be better or worse. It would be interesting to investigate whether immunotherapy with ex-vivo expanded autologous Tregs would be beneficial in patients with HIV progression.
In conclusion, our study is the first to characterize the Helios subsets of Tregs in the paediatric population with perinatal-acquired HIV infection. Within the Treg population, the percentage of Helios+ subset does not fluctuate with CD4 depletion or HIV viraemia but appears to have selective preservation. Future studies will examine why some patients have Helios+ Tregs below 50%, while others are above 80%. A longitudinal study will contribute to a better understanding of the role of Tregs and the Helios subsets.
Acknowledgments
This work was supported by the State of Texas HIV Funds to G. P. H. and Pediatric Department start-up fund to D. Q. T.
Disclosures
The authors have no financial conflicts of interest.
Supporting Information
Fig. S1. Difference in the ratio of regulatory T cells (Treg) and Helios+ Treg to (a) CD8, (b) CD8+CD38+ and (c) CD8+human leucocyte antigen D-related (HLA-DR)+ within the poor, intermediate and good HIV status groups. *P < 0·05; **P < 0·001.
References
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Lozano T, Casares N, Lasarte JJ. Searching for the Achilles heel of FOXP3. Front Oncol. 2013;3:294. doi: 10.3389/fonc.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez AM, Yang Y. The role of natural regulatory T cells in infection. Immunol Res. 2011;49:124–134. doi: 10.1007/s12026-010-8176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta F, Bourgeois C. CD4+FOXP3+ regulatory T-cell subsets in human immunodeficiency virus infection. Front Immunol. 2013;4:215. doi: 10.3389/fimmu.2013.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamichi H, Lane HC. Regulatory T cells in HIV-1 infection: the good, the bad, and the ugly. J Infect Dis. 2012;205:1479–1482. doi: 10.1093/infdis/jis238. [DOI] [PubMed] [Google Scholar]
- Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121:29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLOS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol. 2009;83:12925–12933. doi: 10.1128/JVI.01352-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang L, Wang R, et al. FoxP3+CD4+ regulatory T cells play an important role in acute HIV-1 infection in humanized Rag2−/−gammaC−/− mice in vivo. Blood. 2008;112:2858–2868. doi: 10.1182/blood-2008-03-145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter AL, Hennessey M, Bell A, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004;200:331–343. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D, Jiang Q, Zhang L, Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunol Res. 2008;41:248–266. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Imamichi H, Lane HC. Regulatory T cells in HIV-1 infection: the good, the bad and the ugly. J Infect Dis. 2012;205:1479–1482. doi: 10.1093/infdis/jis238. [DOI] [PubMed] [Google Scholar]
- Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A, Prado JG, Kang YH, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]
- Kared H, Lelievre JD, Donkova-Petrini V, et al. regulatory T cells are associated with higher CD4 cell counts in primary infection. Aids. 2008;22:2451–2460. doi: 10.1097/QAD.0b013e328319edc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- Bi X, Suzuki Y, Gatanaga H, Oka S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur J Immunol. 2009;39:301–309. doi: 10.1002/eji.200838667. [DOI] [PubMed] [Google Scholar]
- Freguja R, Gianesin K, Mosconi I, et al. Regulatory T cells and chronic immune activation in human immunodeficiency virus 1 (HIV-1)-infected children. Clin Exp Immunol. 2011;164:373–380. doi: 10.1111/j.1365-2249.2011.04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand FA, Nixon DF, Loo CP, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLOS ONE. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259:88–102. doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function. Front Immunol. 2013;4:253. doi: 10.3389/fimmu.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouscarat F, Levacher-Clergeot M, Dazza MC, et al. Correlation of CD8 lymphocyte activation with cellular viremia and plasma HIV RNA levels in asymptomatic patients infected by human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1996;12:17–24. doi: 10.1089/aid.1996.12.17. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLOS ONE. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron U, Floess S, Wieczorek G, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- Angin M, Kwon DS, Streeck H, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis. 2012;205:1495–1500. doi: 10.1093/infdis/jis236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta F, Lecuroux C, Girault I, et al. Early and long-lasting alteration of effector CD45RA(−)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis. 2012;205:1510–1519. doi: 10.1093/infdis/jis235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallon NI, Lopez M, Soriano V, et al. Level, phenotype and activation status of CD4+FoxP3+ regulatory T cells in patients chronically infected with human immunodeficiency virus and/or hepatitis C virus. Clin Exp Immunol. 2009;155:35–43. doi: 10.1111/j.1365-2249.2008.03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Xing S, Fu J, Zhang Z, et al. Increased turnover of FoxP3high regulatory T cells is associated with hyperactivation and disease progression of chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2010;54:455–462. doi: 10.1097/QAI.0b013e3181e453b9. [DOI] [PubMed] [Google Scholar]
- Arguello RJ, Balbaryski J, Barboni G, Candi M, Gaddi E, Laucella S. Altered frequency and phenotype of CD4+ forkhead box protein 3+ T cells and its association with autoantibody production in human immunodeficiency virus-infected paediatric patients. Clin Exp Immunol. 2012;168:224–233. doi: 10.1111/j.1365-2249.2012.04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Difference in the ratio of regulatory T cells (Treg) and Helios+ Treg to (a) CD8, (b) CD8+CD38+ and (c) CD8+human leucocyte antigen D-related (HLA-DR)+ within the poor, intermediate and good HIV status groups. *P < 0·05; **P < 0·001.


