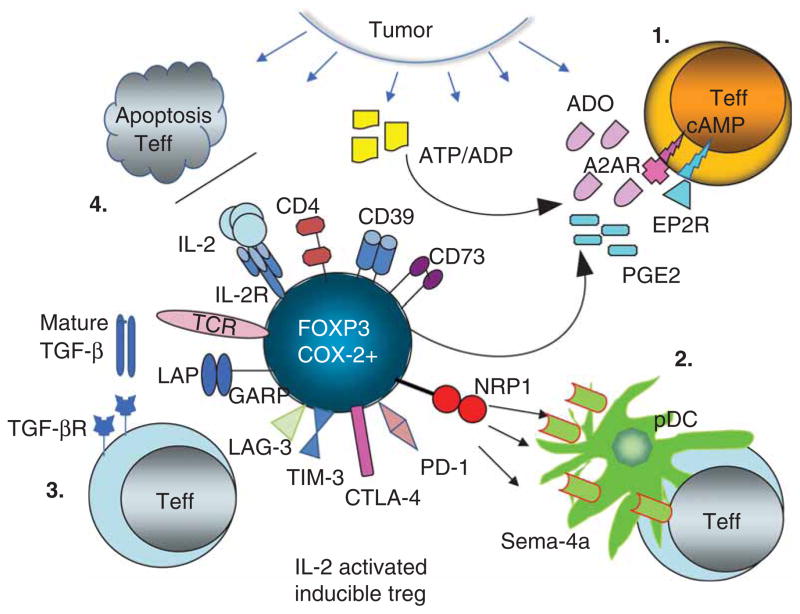
Figure 1. In the TME, activated iTregs operate by engaging several suppressive pathways which downregulate functions or induce apoptosis of immune cells.
Shown are: (1) ADO-PGE2 pathway; (2) the neuropilin1-semaphorin4a pathway; (3) the TGF-β pathway; and (4) the IL-2/IL-2R pathway. Cooperation between these pathways might lead to upregulation of immune-suppressive molecules (e.g., TIM3, PD-1, CTLA-4, LAG-3, CD39, CD73, LAP/GARP) on iTregs. Consumption of IL-2 by iTregs deprives tumor effector cell of growth factors inducing apoptosis. A ligand for NRP1, sema-4a, is expressed on lymphocytes and p-DC as well as tissue cells.
ADO: Adenosine; GARP: Glycoprotein A repetitions predominant; LAP: Latency-associated peptide; NRP1: Neuropilin 1; p-DC: plasmacytoid dendritic cells; PD-1: Programmed cell death; PGE2: Prostaglandin E2; sema-4a: Semaphorin-4a; Teff: Tumor effector cell; TME: Tumor microenvironment.