FIGURE 2.
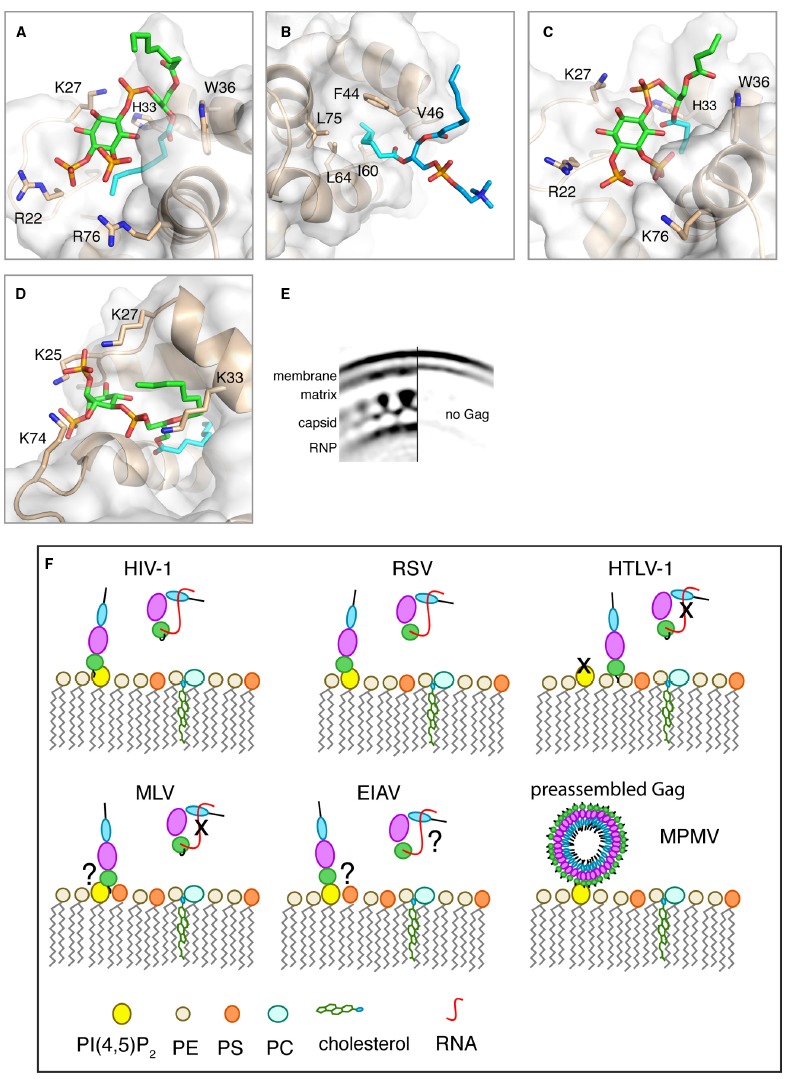
(A–D) Close-up views of structures of HIV-1, HIV-2, and M-PMV MA proteins bound to lipids. MA proteins are shown in ribbon and surface representations with residues implicated in binding shown as sticks. Phospholipids are shown as green [PI(4,5)P2] or blue (PC) sticks and their acyl chains involved in binding are shown in cyan. (A) HIV-1 myr(–)MA bound to di-C8-PI(4,5)P2 (PDB ID: 2H3V). (B) HIV-1 myr(–)MA bound to di-C8-PC (PDB ID: 2LYA). (C) HIV-2 MA bound di-C4-PI(4,5)P2 (PDB ID: 2K4I). (D) M-PMV MA bound to di-C8-PI(4,5)P2 structure provided by Prchal et al. (2012). (E) Cryoelectron microscopy reconstruction of HIV-1 immature particle in a section with (left) or without (right) Gag polyprotein present. Used with permission [Copyright (2009) National Academy of Sciences, USA (Briggs et al., 2009)]. (F) A schematic representation of potential mechanisms for Gag binding to the PM for different retroviruses. Gag binding to membranes is regulated by PI(4,5)P2 and RNA for some but not all retroviruses. Binding of Gag to membranes via the MA domain displaces RNA, which binds non-specifically to the basic region of MA. Data are not in agreement on the role of PI(4,5)P2 in MLV Gag binding to the PM. For EIAV, Gag binding to membranes appears to have no specific requirement for PI(4,5)P2 since other phosphoinositides may also play a role. For M-PMV, Gag assembly occurs in the cytoplasm prior to transport to the PM where MA specifically recognizes PI(4,5)P2.
