Abstract
Recombinant vectors based on adeno-associated virus serotype 8 (AAV8) have been successfully used in the clinic and hold great promise for liver-directed gene therapy. Preexisting immunity against AAV8 or the development of antibodies against the therapeutic transgene product might negatively affect the outcomes of gene therapy. In the prospect of an AAV8-mediated, liver-directed gene therapy clinical trial for mucopolysaccharidosis VI (MPS VI), a lysosomal storage disorder caused by arylsulfatase B (ARSB) deficiency, we investigated in a multiethnic cohort of MPS VI patients the prevalence of neutralizing antibodies (Nab) to AAV8 and the presence of ARSB cross-reactive immunologic material (CRIM), which will either affect the efficacy of gene transfer or the duration of phenotypic correction. Thirty-six MPS VI subjects included in the study harbored 45 (62.5%) missense, 13 (18%) nonsense, 9 (12.5%) frameshift (2 insertions and 7 deletions), and 5 (7%) splicing ARSB mutations. The detection of ARSB protein in 24 patients out of 34 (71%) was predicted by the type of mutations. Preexisting Nab to AAV8 were undetectable in 19/33 (58%) analyzed patients. Twelve out of 31 patients (39%) tested were both negative for Nab to AAV8 and CRIM-positive. In conclusion, this study allows estimating the number of MPS VI patients eligible for a gene therapy trial by intravenous injections of AAV8.
Introduction
Mucopolysaccharidosis VI (MPS VI) or Maroteaux–Lamy syndrome is an autosomal recessive disorder caused by deficiency of the enzyme arylsulfatase B (ARSB), which results in lysosomal storage of the glycosaminoglycan (GAG) dermatan sulfate (DS). Clinical features of the disease include growth retardation, skeletal dysplasia, joint stiffness, organomegaly, heart valve disease, and corneal clouding without primary involvement of the central nervous system.1
Enzyme replacement therapy (ERT) is currently the standard of care for MPS VI.2 However, despite amelioration of both visceral phenotype and endurance, ERT has limited efficacy on several MPS VI complications such as ocular, cardiac, and skeletal abnormalities. In addition, ERT requires inconvenient weekly infusions of costly recombinant enzyme2,3 that are associated with increased risks of adverse reactions such as hypersensitivity reactions.4 Alternative strategies with similar or better therapeutic efficacy without the inconvenience and risks of multiple infusions are desirable.
We have recently demonstrated that a single intravenous administration of a recombinant vector based on adeno-associated virus serotype 8 (AAV2/8), which efficiently transduces liver,5–8 results in long-term therapeutic efficacy in rodent and feline models of MPS VI.9–13 Levels of serum ARSB activity falling within the normal range were detected in MPS VI cats up to 6 years postinjection, which was the last time-point of observation (Ferla and Auricchio, unpublished results). Based on these promising results, translation from bench to bedside of this therapeutic strategy is now ongoing.
The outcome of in vivo gene transfer may be influenced by several factors. Preexisting neutralizing antibodies (Nab) to wild-type AAV8 even at low titers are sufficient to prevent liver transduction by AAV2/8 vectors, and thus significantly reduce the efficacy of gene therapy,14–17 as shown also in MPS VI cats.11 Gene therapy efficacy may also be limited by the development of humoral immunity to the therapeutic transgene product. The immune response is more likely to occur in patients or animal models that carry bi-allelic null mutations in the disease-causative gene because of lack of cross-reactive immunologic material (CRIM). Liver-directed gene transfer with either AAV or lentiviral vectors has been shown to either prevent18 or eradicate19,20 humoral immunity to the transgene product. However, immune tolerization was not observed in MPS VI rats that are homozygous for a null ARSB mutation.9,13
In the prospect of determining patients' eligibility for a clinical trial for MPS VI involving intravenous administrations of AAV2/8 vectors, a multiethnic unselected cohort of MPS VI patients from multiple metabolic clinics has been evaluated for the presence of both Nab to AAV8 and CRIM that will dictate eligibility and will likely affect the outcomes of the clinical trial.
Patients, Materials, and Methods
Patients
The participants in the study were all MPS VI patients diagnosed and followed-up at multiple metabolic clinics in Italy (Catania, Florence, Naples, Monza, and Padua), in the Netherlands (Rotterdam), and in Turkey (Ankara). The clinical research protocol was approved by the Institutional Review Board (IRB) of each participating metabolic clinic. All patients signed an informed consent to be included in the study. The study included the following assessments: (1) clinical and instrumental (height or length; endurance through the 6 min walk test and 3 min stair climb; forced vital capacity; forced expiratory volume at 1 min; joint stiffness and pain, grip, and pinch scores; Childhood Health Assessment Questionnaire scores; visual acuity and ocular abnormalities by full ocular examination; and magnetic resonance imaging of hand, wrist, and knee joints performed in cooperative subjects); (2) biochemical (urinary GAGs, ARSB levels in serum, leucocytes and/or fibroblasts, dosage of Nab to AAV8, clinical chemistry and hematology, and HBV and HCV tests); and (3) molecular (DNA sequencing of the ARSB gene; see below for details).
The molecular and clinical findings of these patients are summarized in Tables 1 and 2. The involvement of the main tissue/organs affected by the disease at the age of last evaluation was graded by the referring clinicians as absent (0), mild (1), moderate (2), or severe (3). A disease severity score ranging from 0 (least severe) to 21 (most severe) was assigned based on the degree of severity of each affected tissue/organ (n=7 tissues/organs).
Table 1.
Arylsulfatase B Mutations in Mucopolysaccharidosis VI Patients
| Patient | Ethnicity | Allele 1 nucleotide/protein change | Allele 2 nucleotide/protein change | Mutations previously reported by |
|---|---|---|---|---|
| 1 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 2 | Turkish | c.908G>A/p.G303E | c.908G>A/p.G303E | Lin et al.42 |
| 3 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 4 | Guinean | c.971G>T/p.G324V | c.971G>T/p.G324V | Karageorgos et al.37 |
| 5 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 6 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 7 | Moroccan | c.883-884dupTT/p.F295FfsX42 | c.883-884dupTT/p.F295FfsX42 | Novel |
| 8 | Turkish | c.903C>G/p.N301K | c.903C>G/p.N301K | Brands et al.23 |
| 9 | Azerbaijan | c.1036delG/p.E346SfsX11 | c.1178A>G/p.H393R | Novel |
| 10 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 11 | Turkish | c.571C>T/p.R191X | c.571C>T/p.R191X | Karageorgos et al.37 |
| 12 | Italian | del exon 5 | del exon 5 | a |
| 13 | Moroccan | c.995T>G/p.V332G | c.995T>G/p.V332G | Brands et al.23 |
| 14 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 15 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 16 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 17 | Turkish | c.571C>T/p.R191X | c.571C>T/p.R191X | Karageorgos et al.37 |
| 18 | Turkish | c.571C>T/p.R191X | c.571C>T/p.R191X | Karageorgos et al.37 |
| 19 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 20 | Venezuelan | c.979C>T/p.R327X | c.979C>T/p.R327X | Karageorgos et al.43 |
| 21 | Dutch | c.629A>G/p.Y210C | c.979C>T/p.R327X | Litjens et al.26 and Karageorgos et al.43 |
| 22 | Italian | c.943C>T/p.R315X | c.1475delC/pP492LfsX80 | Voskoboeva et al.44 Di Natale et al. |
| 23 | Italian | c.943C>T/p.R315X | c.899-1142del | Arlt et al.45 and Voskoboeva et al.44 |
| 24 | Turkish | c.962T>C/p.L321P | c.962T>C/p.L321P | Isbrandt et al.41 |
| 25 | Dutch | c.629A>G/p.Y210C | c.979C>T/p.R327X | Litjens et al.26 and Karageorgos et al.43 |
| 26 | Dutch | c.629A>G/p.Y210C | c.979C>T/p.R327X | Litjens et al.26 and Karageorgos et al.43 |
| 27 | Pakistani | c.1142+2T>C | c.1142+2T>C | Brands et al.23 |
| 28 | Turkish | c.454C>T/p.R152W | c.454C>T/p.R152W | Voskoboeva et al.46 |
| 29 | Turkish | c.454C>T/p.R152W | c.454C>T/p.R152W | Voskoboeva et al.46 |
| 30 | Dutch | c.629A>G/p.Y210C | c.937C>G/p.P313A | Litjens et al.26 and Brooks et al.47 |
| 31 | Italian | c.1213+6T>C | c.725A>C/p.H242P | Di Natale et al.24 |
| 32 | Turkish | c.1168G>A/p.E390K | c.1168G>A/p.E390K | Kantaputra et al.48 |
| 33 | Turkish | c.1036delG/p.E346SfsX11 | c.1036delG/p.E346SfsX11 | Novel |
| 34 | Italian | c.1213+6T>C | c.1213+6T>C | Di Natale et al.24 |
| 35 | Turkish | c.454C>T/p.R152W | c.454C>T/p.R152W | Voskoboeva et al.46 |
| 36 | Turkish | c.1168G>A/p.E390K | c.1168G>A/p.E390K | Kantaputra et al.48 |
Patient 12 (BA1196) carries a genomic deletion of exon 5 on both arylsulfatase B (ARSB) alleles; the breakpoints of the deletion were not defined at the nucleotide levels.
Table 2.
Clinical Severity, Cross-Reactive Immunologic Material Status, and Anti–Adeno-Associated Virus Serotype 8 Antibody Titers in Mucopolysaccharidosis VI Patients
| Involvement | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age at the last evaluation (years)/gender | Age at diagnosis (years) | Age at start of ERT (years) | Eye | ENT | Heart | Growth | Bone and joints | Spinal cord | Motor | Clinical score (0–24) | CRIM | Nab anti-AAV8 |
| 1 | 5/M | 1.0 | 1.0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 8 | + | n.a. |
| 2 | 5/M | 1.0 | 2.0 | 1 | 0 | 2 | 1 | 2 | 0 | 2 | 8 | + | <5 |
| 3 | 5/F | 4.0 | 4.0 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 8 | n.a | <5 |
| 4 | 6/M | 1.9 | 2.3 | 2 | 1 | 1 | 1 | 2 | 0 | 1 | 8 | + | 160 |
| 5 | 7/M | 2.5 | 3.0 | 1 | 1 | 1 | 2 | 2 | 0 | 2 | 9 | + | 5 |
| 6 | 7/F | 4.0 | 4.0 | 1 | 2 | 3 | 2 | 2 | 0 | 2 | 12 | + | 40 |
| 7 | 7/M | 1.7 | 2.5 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 8 | − | <5 |
| 8 | 7/F | 1.8 | 2.1 | 2 | 1 | 2 | 3 | 2 | 0 | 2 | 12 | + | <5 |
| 9 | 8/F | 4.5 | n.a | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 10 | + | n.a |
| 10 | 8/M | 3.0 | 3.0 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 9 | + | <5 |
| 11 | 8/M | 2.0 | 4.0 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 12 | − | 10 |
| 12 | 8/M | 0.6 | 2.0 | 1 | 3 | 1 | 3 | 3 | 2 | 2 | 15 | − | <5 |
| 13 | 9/F | 2.8 | 2.9 | 2 | 0 | 2 | 2 | 2 | 1 | 2 | 11 | + | <5 |
| 14 | 10/F | 2.0 | 3.5 | 2 | 1 | 2 | 2 | 3 | 3 | 2 | 15 | + | <5 |
| 15 | 10/M | 1.0 | 4.0 | 2 | 1 | 2 | 2 | 3 | 2 | 2 | 14 | + | <5 |
| 16 | 12/M | 2.0 | 5.0 | 3 | 1 | 2 | 2 | 2 | 0 | 2 | 12 | + | 10 |
| 17 | 12/M | 7.5 | 7.5 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 12 | − | 20 |
| 18 | 12/M | 8.0 | 8.0 | 2 | 1 | 2 | 2 | 3 | 0 | 2 | 12 | − | 20 |
| 19 | 12/F | 5.0 | 6.0 | 2 | 1 | 2 | 2 | 1 | 0 | 2 | 10 | + | n.a |
| 20 | 12/M | 5.0 | 5.4 | 2 | 2 | 1 | 3 | 3 | 3 | 3 | 17 | − | <5 |
| 21 | 12/M | 5.8 | 6.1 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 5 | + | 20 |
| 22 | 12/M | 1.2 | 6.1 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 18 | n.a | <5 |
| 23 | 12/M | 1.6 | 4.0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 13 | − | <5 |
| 24 | 13/F | 4.0 | 7.0 | 3 | 2 | 2 | 2 | 1 | 2 | 2 | 14 | + | 5 |
| 25 | 13/M | 5.1 | 5.9 | 0 | 0 | 1 | 2 | 2 | 0 | 3 | 8 | + | <5 |
| 26 | 13/F | 7.4 | 7.8 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 6 | + | 640 |
| 27 | 13.5/F | 3.4 | 6.8 | 2 | 2 | 3 | 3 | 3 | 0 | 3 | 16 | − | <5 |
| 28 | 14/F | 7.8 | 8.3 | 1 | 1 | 1 | 2 | 2 | 0 | 1 | 8 | + | <5 |
| 29 | 14/M | 0.7 | 7.6 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 5 | + | <5 |
| 30 | 14/M | 10.2 | 10.6 | 0 | 0 | 1 | 1 | 2 | 0 | 3 | 7 | + | <5 |
| 31 | 14/F | 4.0 | 6.0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 5 | + | <5 |
| 32 | 17/M | 12 | 13.0 | 0 | 1 | 2 | 2 | 3 | 3 | 2 | 13 | + | 20 |
| 33 | 17/M | 3.5 | 11.5 | 2 | 3 | 2 | 2 | 3 | 2 | 2 | 16 | − | 160 |
| 34 | 21/F | 4.5 | 10.0 | 2 | 2 | 3 | 3 | 3 | 1 | 2 | 16 | − | 320 |
| 35 | 23/M | 10.1 | 18.3 | 0 | 1 | 1 | 2 | 2 | 0 | 3 | 9 | + | <5 |
| 36 | 25/F | 23 | 24 | 0 | 1 | 2 | 2 | 3 | 2 | 2 | 12 | + | 20 |
CRIM, cross-reactive ARSB immunologic material; ENT, ear, nose, and throat; ERT, enzyme replacement therapy; F, female; M, male; n.a, not available.
Involvement was graded as absent (0), mild (1), moderate (2), or severe (3).
Cell culture
Cultured skin fibroblasts from MPS VI patients obtained for diagnostic purposes were maintained in Dulbecco's modified Eagle's medium (Gibco–Life Invitrogen, Paisley, UK) containing 20% fetal bovine serum (Gibco, Invitrogen Corporation, Carlsbad, CA), 1% of Glutamine (Sigma-Aldrich, Steinheim, Germany), and 1% antibiotic–antimycotic (Gibco, Invitrogen Corporation).
Mutational analysis of the ARSB gene
Genomic DNA was extracted from cultured skin fibroblasts by phenol/chloroform extraction. All ARSB exons were amplified by PCR, using M13-tailed primers. Sanger sequencing was performed with an ABI PRISM 3130 XL automatic DNA Sequencer Genetic Analyzer (Applied Biosystems, Foster City, CA). Primer sequences and PCR conditions are described in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/hum).
ARSB cross-reactive immunologic material analysis
The presence of ARSB cross-reactive immunologic material was evaluated by Western blot analysis of cultured skin fibroblast lysates. Cells were lysed in RIPA buffer (50 mM NaCl, 25 mM Tris-HCl pH 8.0, 0.5% NP40, 0.1% SDS, and 10 mg/ml leupeptin–aprotinin–pepstatin A). Seventy micrograms of proteins were separated by SDS-PAGE on a 10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (Roche, Basel, Switzerland), and incubated with the following antibodies: (1) sheep polyclonal anti-ARSB antibody, diluted to 1:500 in 5% milk, prepared as previously described,21 and (2) mouse monoclonal anti-beta actin antibody (A5441; Sigma-Aldrich, Milan, Italy), diluted to 1:3000 in 5% nonfat dried milk (Euroclone, Milan, Italy).
Nab to AAV assay
MPS VI patients were screened for preexisting Nab to AAV8 using an in vitro transduction inhibition assay with Huh7 cells, as previously described.22 The detection limit of the assay is a 1:5 serum dilution.
Results
ARSB mutations and ARSB cross-reactive immunologic material in MPS VI patients
Serum and skin fibroblasts from 36 patients with a clinical diagnosis of MPS VI confirmed by ARSB deficiency on enzyme assay were collected from referral metabolic clinic centers in Italy, the Netherlands, and Turkey, which provided samples from 7, 11, and 18 MPS VI patients, respectively (Tables 1 and 2). The age of patients ranged from 5 to 25 years (age 5–10, n=15; age 11–18, n=18; age 19–25, n=3; Table 1) and their severity varied significantly (Table 2).
At the time of this study, molecular diagnoses of MPS VI were already available for 18 out of the 36 patients.23–25 In the remaining 18 patients, PCR amplification and Sanger sequencing of all ARSB exons and intron–exon boundaries were carried out. Collectively, we observed 45/72 (62.5%) missense, 13/72 (18%) nonsense, 9/72 (12.5%) frameshift (2 insertions and 7 deletions), and 5/72 (7%) splicing ARSB mutations (Table 1). To the best of our knowledge, three mutations had not been previously described. These were one missense (c.1178 A>G p.H393R), not reported in dbSNP or in exome server databases, resulting in the change of a highly conserved histidine residue (Supplementary Fig. S1) that had already been found to be mutated in MPS VI,26 and two frameshift mutations (883-884duplTT [p.F295FfsX42], c.1036delG [p.E346SfsX11], Table 1) predicted to result in truncated proteins.
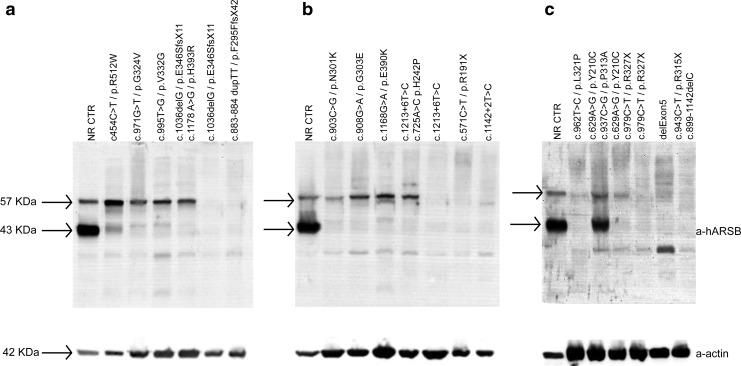
Genotypes of MPS VI patients were correlated with the presence of ARSB CRIM by Western blot analysis with anti-ARSB antibody of lysates from cultured skin fibroblasts (Fig. 1). In 24/34 (71%) of analyzed patients, 43 and 57 kDa bands corresponding to ARSB mature and precursor proteins, respectively, were detected (Fig. 1). Based on this observation, these MPS VI patients were deemed CRIM positive. Importantly, the CRIM status could be predicted based on ARSB mutations; all patients bearing missense mutations were consistently found to be CRIM positive, whereas patients bearing null mutations in both alleles were CRIM negative (Tables 1 and 2). No correlation between clinical severity and CRIM status was observed (Fisher exact test p-value=0.2). However, the power of the test was low (0.03) because of the small sample size, and thus definitive conclusions on this aspect cannot be drawn.
FIG. 1.
ARSB CRIM in MPS VI patients. The presence of ARSB CRIM was evaluated by Western blot analysis of lysates of cultured skin fibroblasts, as described in the Patients, Materials, and Methods section. Samples corresponding to each ARSB genotype have been loaded in (a–c). Bands of 43 and 57 kDa corresponding to ARSB mature and precursor proteins, respectively, are indicated by the arrows. ARSB, arylsulfatase B; CRIM, cross-reactive immunologic material; MPS, mucopolysaccharidosis; NR CTR, normal control. The following mutations have not been described before: c.1178 A>G (p.H393R), 883-884duplTT (p.F295FfsX42), and c.1036delG (p.E346SfsX11).
Evaluation of Nab to AAV8 in MPS VI patients
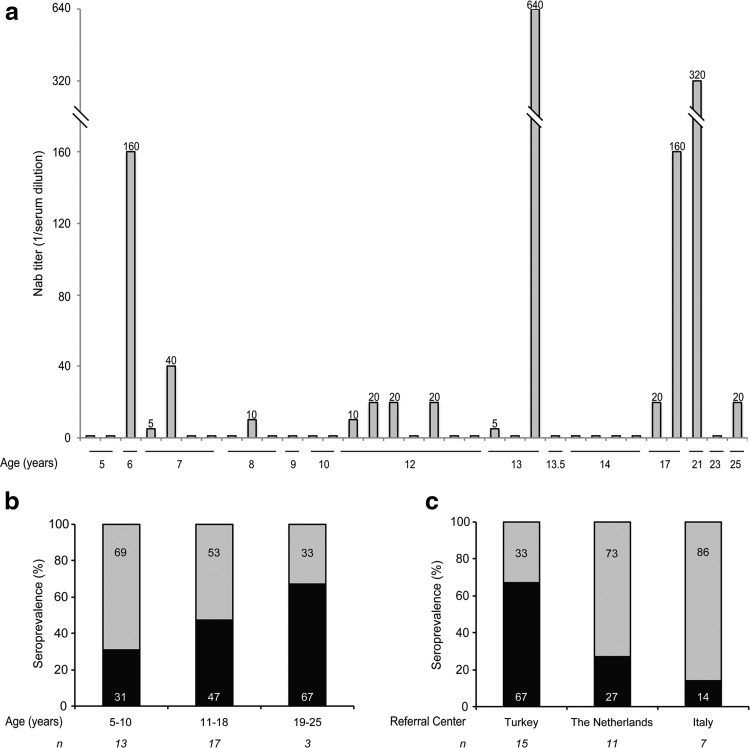
Sera from MPS VI patients were analyzed using an in vitro AAV2/8 transduction inhibition assay22 to detect Nab to AAV8. Nab to AAV8 were undetectable in the sera of 19/33 of analyzed patients (58%), whereas sera from the remaining 14 patients (42%) showed a titer of Nab ≥1:5, which is the detection limit of the assay (Table 2). The seroprevalence was 31%, 47%, and 67% in patients whose age ranged between 5 and 10, 11 and 18, and 19 and 25 years, respectively (Fig. 2a and b). The highest seroprevalence (67%) was found in patients from the referral center in Turkey, followed by patients from the Dutch (27%) and Italian (14%) referral centers (Fig. 2c). Statistical analysis (Fisher exact test) suggested no association between Nab and age (p=0.5), and, conversely, a significant difference between Nab and referral centers (p=0.05). However, the power of the test was low (0.2 and 0.5, respectively) because of the small sample size. Hence, definitive conclusions on these aspects cannot be inferred.
FIG. 2.
Seroprevalence of Nab to AAV8 by age and referral centers. The Nab titer of each patient is reported in (a). Data relative to Nab to AAV8 in MPS VI patients were additionally stratified by age (b) and referral center (c). The percentage of samples positive (Nab +, black bar) and negative (Nab −, gray bar) for Nab to AAV8 is reported in (b) and (c), and the corresponding values are inside each bar. The number (n) of patients in each group is indicated under each bar. AAV8, adeno-associated virus serotype 8; Nab, neutralizing antibodies.
Discussion
Gene therapy is emerging as a successful strategy for treatment of inherited diseases.27 In vivo gene transfer with AAV2/8 vectors is regarded as a possible approach to convert the liver into a factory organ for systemic release of therapeutic proteins. A recent clinical trial using intravenous administrations of AAV2/8 in patients with hemophilia B has supported this paradigm in humans; some treated patients exhibited multiyear stable factor IX levels following a single administration of vector.15,28 By a similar approach, we have recently demonstrated that a single systemic administration of AAV2/8 encoding ARSB is able to convert the liver into a factory organ, providing stable secretion of ARSB. This results in long-term therapeutic efficacy in rodent and feline models of MPS VI.9–13 Gene therapy was at least as effective as weekly infusions of recombinant human ARSB (rhARSB) in a mouse model of MPS VI,12 suggesting that it may be preferable to ERT, especially because it potentially requires a single intravenous administration. This bodes well for further investigating the safety and efficacy of intravenous administrations of AAV2/8 in patients with MPS VI.
Immune responses to the vector have been shown to limit both efficacy and longevity of gene transfer.27,29 Very low levels of Nab to AAV8 are sufficient to strongly reduce liver transduction and thus therapeutic efficacy.11,14,16,17 Decoy capsids that inhibit circulating Nab without interfering with endogenous AAV receptors and AAV preparations with empty capsids are two potential strategies to overcome the barrier of preexisting anti-AAV antibodies.30 However, until these strategies are demonstrated to be safe and efficient in humans, the presence of circulating Nab to AAV8 is better considered an exclusion criterion in trials in which systemic administrations of AAV2/8 vectors are used. Therefore, we screened MPS VI patients for Nab to AAV8, which were undetectable in 58% of subjects. Among the MPS VI patients tested for Nab to AAV8, the titer was >1:20 in 15% and >1:80 in 12% of subjects. These results are consistent with previously published data showing that the seroprevalence of Nab to AAV8 at serum dilution of >1:20 and >1:80 is about 20% and 15%, respectively, in samples from healthy European individuals.22,31 The seroprevalence of Nab to AAV8 observed in our cohort is slightly lower than that (33%) reported in both healthy subjects and hemophilic patients from Japan.32
No definitive conclusions can be drawn from the analyses of AAV8 seroprevalence stratified by referral centers, age, or clinical severity because of the small sample size. However, we have observed that AAV8 seroprevalence varies between samples collected from different referral centers and is highest in patients of Asiatic origin (50%) followed by patients of European and African origins (33% each). These observations support previous studies in which AAV seroprevalence was shown to vary depending on the geographical origin of the studied population; this was probably at least partially attributed to living conditions or population density.22,33 In line with a previous study showing that seroprevalence to AAV8 significantly increases through the childhood and the adolescence in healthy subjects,34 the seroprevalence to AAV8 we have measured is higher in adolescents than in children and does not correlate with the clinical severity of the disease (Fisher exact test p-value=0.95; power 0.06), which may account for an increased exposure because of more frequent clinic or hospital admissions.
Gene therapy efficacy may be additionally limited by T-cell-mediated and humoral immunity to the therapeutic transgene product. T-cell-mediated responses to rhARSB have not been reported in MPS VI patients treated with ERT.2,35 On the other hand, recent results from phase I/II liver gene therapy clinical trials for hemophilia B and acute intermittent porphyria showed no T-cell-mediated immune responses to the therapeutic transgene product,15,36 suggesting that this might not be a major limitation for liver gene therapy.
The risk of humoral immunity to the transgene product correlates with the presence of CRIM.9,13 Here, we show that the CRIM positivity of MPS VI patients could be precisely predicted by the presence of at least one ARSB allele carrying a missense mutation. To the best of our knowledge, this is the first example of analysis, which correlates the genotype of MPS VI patients with ARSB CRIM, clearly showing that the CRIM status could be predicted based on ARSB mutations. On the contrary, there was no correlation between CRIM status and disease severity. The frequencies of ARSB missense and null mutations that we found in our series of MPS VI patients (62.5% and 37.5%, respectively) are similar to those previously found in a larger patient cohort.37
While even low levels of Nab to AAV8 should be viewed as an exclusion criterion for a trial with AAV2/8 infusions, exclusion of ARSB CRIM-negative MPS VI patients is debatable. First, almost all MPS VI patients treated with ERT develop antibodies to rhARSB independently of their genotypes or disease severity.4,23,38–40 Nevertheless, they experience clinical benefits under ERT.4,38–40 In addition, liver-directed gene therapy has been demonstrated to either prevent the generation of humoral immunity18 or to eradicate it, if already present,19,20 which makes the impact of preexisting humoral immunity to the transgene product on gene therapy efficacy difficult to predict. Therefore, there is a good argument for not excluding neither patients with preexisting immunity to rhARSB nor ARSB CRIM-negative patients from the trial.
Although CRIM-negative status would not be an exclusion criterion, CRIM-positive patients may be preferred in a gene therapy trial as they have lower possibility to generate humoral immunity to the newly synthesized transgene product secreted by transduced hepatocytes.9,13 The current study has revealed that 12 of 19 patients negative for Nab to AAV8 were also CRIM positive. These 12 patients correspond to 39% of the patients analyzed for both markers. Although using both criteria will likely result in a significant reduction of eligible MPS VI subjects, based on the estimated number from this study, a phase I/II, open-label, dose-escalation (2 patients treated at a low, 3 at an intermediate, and 3 at a high dose of vector) safety study would still be feasible.
Supplementary Material
Acknowledgments
We gratefully acknowledge Lucia Perone (Cell Culture and Cytogenetics Core, Telethon Institute of Genetics and Medicine [TIGEM], Pozzuoli, Naples) for culturing of MPS VI fibroblasts; Annamaria Carissimo and Luisa Cutillo (Bioinformatics core, TIGEM, Pozzuoli, Naples) for statistical analysis; Alexis Burton (Scientific Office, TIGEM, Pozzuoli, Naples) for critical reading of this article; Roberto Calcedo (Gene Therapy Program, University of Pennsylvania) for Nab analysis; and Bianca Fontanella (Scientific and Regulatory Office, TIGEM, Pozzuoli, Naples) and Valentina Pecorella (Clinical Trial Office, Section of Pediatrics, Department of Translational Medicine, “Federico II” University, Naples) for data collection from referral centers.
This work was supported by the European Community's Seventh Framework Programme (FP7/2007–2013)—MeuSIX [304999] and by the Isaac Foundation.
Author Disclosure Statement
Rita Ferla, Pamela Claudiani, Marco Savarese, Giancarlo Parenti, Simona Fecarotta, Vincenzo Nigro, Ans Van Der Ploeg, Hatice Serap Sivri, Generoso Andria, Nicola Brunetti-Pierri, and Alberto Auricchio declare that no competing financial interests exist.
Karen Kozarsky has no conflicts of interest beyond being an employee of REGENXBIO.
References
- 1.Neufeld E, Muenzer J. The mucopolysaccharidoses. In: The Online Metabolic and Molecular Bases of Inherited Disease. Scriver R, ed. (McGraw Hill, New York: ). 2010; pp. 1–73 [Google Scholar]
- 2.Giugliani R, Lampe C, Guffon N, et al. . Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)-10-year follow-up of patients who previously participated in an MPS VI survey study. Am J Med Genet A 2014;164A:1953–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyatt K, Henley W, Anderson L, et al. . The effectiveness and cost-effectiveness of enzyme and substrate replacement therapies: a longitudinal cohort study of people with lysosomal storage disorders. Health Technol Assess 2012;16:1–543 [DOI] [PubMed] [Google Scholar]
- 4.Harmatz P, Giugliani R, Schwartz I, et al. . Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr 2006;148:533–539 [DOI] [PubMed] [Google Scholar]
- 5.Cotugno G, Annunziata P, Barone MV, et al. . Impact of age at administration, lysosomal storage, and transgene regulatory elements on AAV2/8-mediated rat liver transduction. PLoS One 2012;7:e33286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao GP, Alvira MR, Wang L, et al. . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani AC, Rosales C, McIntosh J, et al. . Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011;19:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Wang H, Bell P, et al. . Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther 2010;18:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotugno G, Tessitore A, Capalbo A, et al. . Different serum enzyme levels are required to rescue the various systemic features of the mucopolysaccharidoses. Hum Gene Ther 2010;21:555–569 [DOI] [PubMed] [Google Scholar]
- 10.Cotugno G, Annunziata P, Tessitore A, et al. . Long-term amelioration of feline Mucopolysaccharidosis VI after AAV-mediated liver gene transfer. Mol Ther 2011;19:461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferla R, O'Malley T, Calcedo R, et al. . Gene therapy for mucopolysaccharidosis type VI is effective in cats without pre-existing immunity to AAV8. Hum Gene Ther 2013;24:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferla R, Claudiani P, Cotugno G, et al. . Similar therapeutic efficacy between a single administration of gene therapy and multiple administrations of recombinant enzyme in a mouse model of lysosomal storage disease. Hum Gene Ther 2014;25:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tessitore A, Faella A, O'Malley T, et al. . Biochemical, pathological, and skeletal improvement of mucopolysaccharidosis VI after gene transfer to liver but not to muscle. Mol Ther 2008;16:30–37 [DOI] [PubMed] [Google Scholar]
- 14.Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 15.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Calcedo R, Wang H, et al. . The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther 2010;18:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Calcedo R, Bell P, et al. . Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther 2009;9:104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annoni A, Cantore A, Della Valle P, et al. . Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol Med 2013;5:1684–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markusic DM, Hoffman BE, Perrin GQ, et al. . Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies. EMBO Mol Med 2013;5:1698–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meikle PJ, Grasby DJ, Dean CJ, et al. . Newborn screening for lysosomal storage disorders. Mol Genet Metab 2006;88:307–314 [DOI] [PubMed] [Google Scholar]
- 22.Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brands MM, Hoogeveen-Westerveld M, Kroos MA, et al. . Mucopolysaccharidosis type VI phenotypes-genotypes and antibody response to galsulfase. Orphanet J Rare Dis 2013;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Natale P, Villani GR, Parini R, et al. . Molecular markers for the follow-up of enzyme-replacement therapy in mucopolysaccharidosis type VI disease. Biotechnol Appl Biochem 2008;49:219–223 [DOI] [PubMed] [Google Scholar]
- 25.Villani GR, Grosso M, Pontarelli G, et al. . Large deletion involving exon 5 of the arylsulfatase B gene caused apparent homozygosity in a mucopolysaccharidosis type VI patient. Genet Test Mol Biomarkers 2010;14:113–120 [DOI] [PubMed] [Google Scholar]
- 26.Litjens T, Brooks DA, Peters C, et al. . Identification, expression, and biochemical characterization of N-acetylgalactosamine-4-sulfatase mutations and relationship with clinical phenotype in MPS-VI patients. Am J Hum Genet 1996;58:1127–1134 [PMC free article] [PubMed] [Google Scholar]
- 27.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 28.Davidoff AM. AAV-8 Mediated Gene Therapy for Hemophilia B—Oral Communication. American Society of Cell and Gene Therapy, Washington, DC, 2014 [Google Scholar]
- 29.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013;122:23–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mingozzi F, Anguela XM, Pavani G, et al. . Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med 2013;5:194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 32.Mimuro J, Mizukami H, Shima M, et al. . The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J Med Virol 2014;86:1990–1997 [DOI] [PubMed] [Google Scholar]
- 33.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calcedo R, Morizono H, Wang L, et al. . Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011;18:1586–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harmatz P, Giugliani R, Schwartz IV, et al. . Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab 2008;94:469–475 [DOI] [PubMed] [Google Scholar]
- 36.D'Avola D, Lopez-Franco E, Harper P, et al. . Phase I clinical trial of liver directed gene therapy with rAAV5/2-PBGD in acute intermittent porphyria: safety data. European Society of Cell and Gene Therapy Abstract OR036, 2014 [Google Scholar]
- 37.Karageorgos L, Brooks DA, Pollard A, et al. . Mutational analysis of 105 mucopolysaccharidosis type VI patients. Hum Mutat 2007;28:897–903 [DOI] [PubMed] [Google Scholar]
- 38.Harmatz P, Whitley CB, Waber L, et al. . Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J Pediatr 2004;144:574–580 [DOI] [PubMed] [Google Scholar]
- 39.Harmatz P, Ketteridge D, Giugliani R, et al. . Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics 2005;115:e681–e689 [DOI] [PubMed] [Google Scholar]
- 40.Harmatz P, Kramer WG, Hopwood JJ, et al. . Pharmacokinetic profile of recombinant human N-acetylgalactosamine 4-sulphatase enzyme replacement therapy in patients with mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): a phase I/II study. Acta Paediatr 2005;94:61–68; discussion 57. [DOI] [PubMed] [Google Scholar]
- 41.Isbrandt D, Arlt G, Brooks DA, Hopwood JJ, et al. . Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): six unique arylsulfatase B gene alleles causing variable disease phenotypes. Am J Hum Genet 1994;54:454–463 [PMC free article] [PubMed] [Google Scholar]
- 42.Lin WD, Lin SP, Wang CH, Hwu WL, et al. . Genetic analysis of mucopolysaccharidosis type VI in Taiwanese patients. Clin Chim Acta 2008;394:89–93 [DOI] [PubMed] [Google Scholar]
- 43.Karageorgos L, Harmatz P, Simon J, Pollard A, et al. . Mutational analysis of mucopolysaccharidosis type VI patients undergoing a trial of enzyme replacement therapy. Hum Mutat 2004;23:229–233 [DOI] [PubMed] [Google Scholar]
- 44.Voskoboeva E, Krasnopol'skaia KD, Peters K, von Figura K. [Identification of mutations in the arylsulfatase B gene in Russian mucopolysaccharidosis type VI patients]. Genetika 2000;36:837–843 [PubMed] [Google Scholar]
- 45.Arlt G, Brooks DA, Isbrandt D, Hopwood JJ, et al. . Juvenile form of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). A C-terminal extension causes instability but increases catalytic efficiency of arylsulfatase B. J Biol Chem 1994;269:9638–9643 [PubMed] [Google Scholar]
- 46.Voskoboeva E, Isbrandt D, von Figura K, Krasnopolskaya X, et al. . Four novel mutant alleles of the arylsulfatase B gene in two patients with intermediate form of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Hum Genet 1994;93:259–264 [DOI] [PubMed] [Google Scholar]
- 47.Brooks DA, Gibson GJ, Karageorgos L, Hein LK, et al. . An index case for the attenuated end of the mucopolysaccharidosis type VI clinical spectrum. Mol Genet Metab 2005;85:236–238 [DOI] [PubMed] [Google Scholar]
- 48.Kantaputra PN, Kayserili H, Guven Y, Kantaputra W, et al. . Clinical manifestations of 17 patients affected with mucopolysaccharidosis type VI and eight novel ARSB mutations. Am J Med Genet A 2014;164A:1443–1453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.