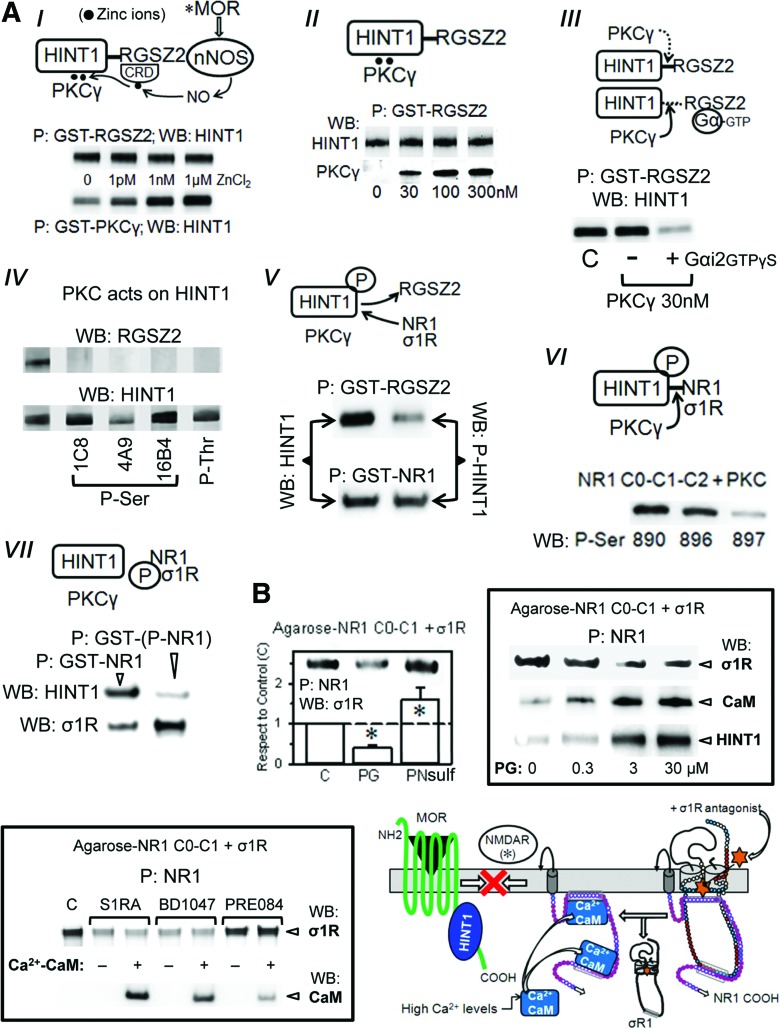
FIG. 7.
The redox-regulated coupling of NMDARs with MORs: effect of σ1R ligands. (A) MOR-mediated activation of NMDARs: (I) Zinc-mediated association of PKCγ with the HINT1 protein. Zinc was removed from the recombinant proteins (TPEN-EDTA buffer) before incubation of 200 nM HINT1 with 100 nM GST-PKCγ in presence of ZnCl2. GST alone did not bind to the HINT1 protein. CRD stands for cysteine-rich domain and P for precipitation of the GST protein with GS. A similar study was conducted between 100 nM GST-RGSZ2 and HINT1. (II) The RGSZ2, PKCγ, and HINT1 form a ternary complex: 200 nM HINT1 were incubated with 100 nM GST-RGSZ2 in the presence of 100 nM ZnCl2 and increasing concentrations of PKCγ. Details as in (I). (III) PKCγ disrupts HINT1-RGSZ2 association in presence of GαGTPγS subunits. GST-RGSZ2 (100 nM) and HINT1 (200 nM) were incubated in the absence or presence of 100 nM Gαi2GTPγS and of PKCγ. (IV) Effect of PKCγ on RGSZ2 and HINT1. RGSZ2 (100 nM) or HINT1 (100 nM) were incubated for 20 min at RT with 30 nM PKCγ. The PKCγ-induced phosphorylation of RGSZ2 and HINT1 was evaluated using specific anti-phospho antibodies. (V) Effect of HINT1 phosphorylation on its association with RGSZ2 and the C-terminal cytosolic region of NR1 subunit (C0-C1-C2). Native and PKC-phosphorylated HINT1 proteins (200 nM) were incubated with either 100 nM GST-RGSZ2 or GST-NR1. (VI) Phosphorylation of NR1 C0-C1-C2 by PKC, and (VII) its association with HINT1 and σ1R. Further details in Materials and Methods section. (B) Left: Effect of PG and PN sulfate on the association σ1R-NR1 C0-C1-C2. Agarose-NR1 was preincubated in the presence of 2.5 mM CaCl2 (30 min, RT) with the σ1R (100 nM) before the addition of 30 μM PG or PN (30 min, RT). P indicates that agarose was recovered and washed before the analysis of the NR1-bound σ1R through SDS-PAGE and WB. Each bar represents the mean±SEM of three determinations using different gels and blots. *Significant differences with respect the control without neurosteroid, ANOVA-Student–Newman–Keuls test; p<0.05. Right: Influence of increasing concentrations of PG on the association of NR1 with σ1Rs. Agarose-NR1 was incubated with the σ1R before the addition of PG. Agarose-NR1 carrying the associated σ1Rs was recovered and washed before the addition of 200 nM HINT1 or 100 nM CaM (in the presence of 2.5 mM CaCl2 and of 30 μM peptide 4). Low: This assay was also conducted with σ1R antagonists (S1RA and BD1047) and the agonist PRE084 in the absence or presence of CaM. Diagram: S1RA removes the σ1R from the NR1 subunit, favoring the binding of Ca2+-CaM, which impairs the interaction of NMDARs with MOR-HINT1 complexes. As a result, morphine only recruits a fraction of the NMDAR activity that is required to control its effects. Amino acid charge, see key in Fig. 3C. GS, glutathione-sepharose; WB, Western blotting. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars