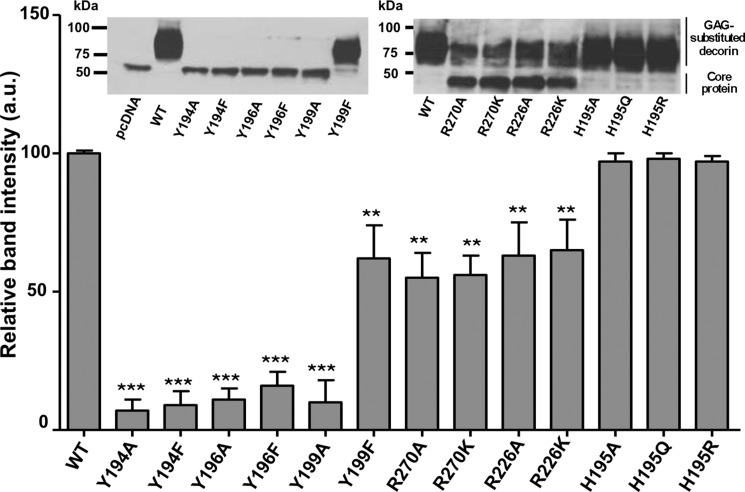
FIGURE 3.
Effect of wild-type and mutated hβ4GalT7 expression on decorin core protein glycosylation in CHOpgsB-618 cells. Cells stably expressing the human recombinant decorin core protein were transfected with the recombinant vector encoding either the wild-type (WT) or mutated hβ4GalT7. The decorin glycosylation level was monitored by immunoblot then quantified using ImageJ software, as described under “Experimental Procedures.” Immunoblot analyses of the decorin core protein glycanation level are shown as inserts for CHOpgsB-618 cells transfected with empty (pcDNA) or with the recombinant vector coding for WT, Y194A, Y194F, Y196A, Y196F, Y199A, or Y199F hβ4GalT7 (left panel), and for WT, R270A, R270K, R226A, R226K, H195A, H195Q, or H195R hβ4GalT7 (right panel). The band observed at a molecular mass of ∼35 kDa can be attributed to the decorin core protein, whereas the wide upper band corresponding to a molecular mass ≥75 kDa corresponds to the glycosylated decorin. Data are mean ± S.E. from three independent experiments performed in triplicate. Statistical analysis was carried out by the Student's t test with **, p < 0.01 and ***, p < 0.001 versus decorin glycosylation in cells expressing the wild-type hβ4GalT7.