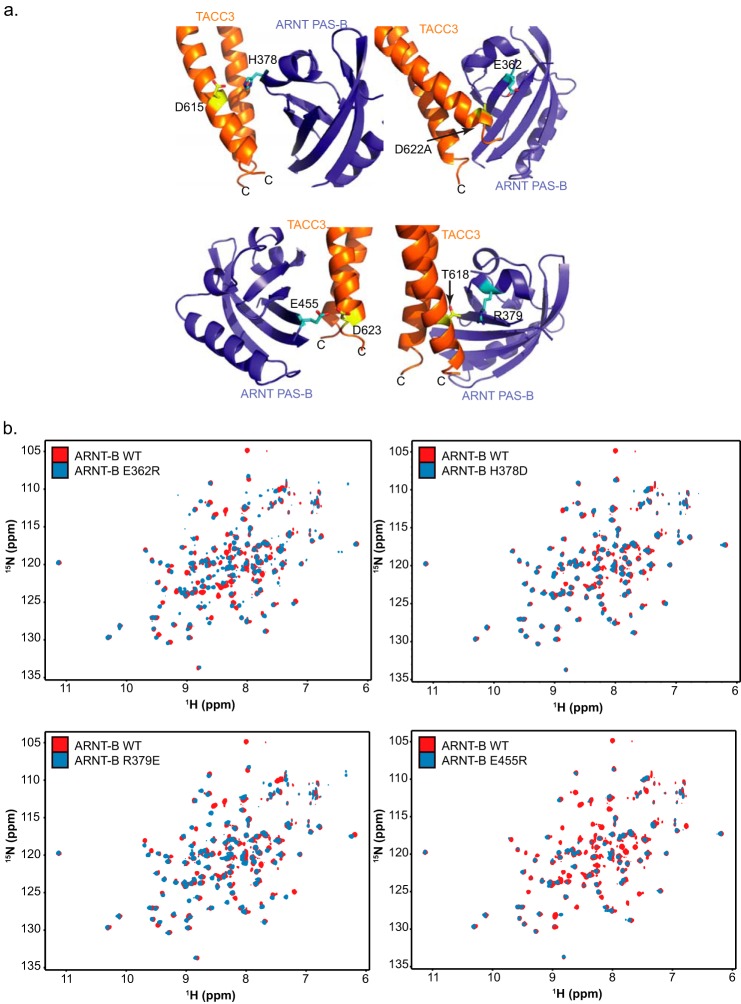
FIGURE 3.
Illustration of four key residue pairs at the ARNT PAS-B·TACC3 interface. a, several pairs of residues at the ARNT·TACC3 interface involve charged groups; changing the electrostatic status of these residues can affect the ARNT PAS-B·TACC3 interaction (Fig. 2, c and e). b, 15N/1H HSQC spectra of four key ARNT PAS-B mutants shown in a confirm that these mutants are not unfolded by the mutations and adopt similar structures to the wild-type proteins, as established by good chemical shift dispersion and overlap with the WT ARNT PAS-B spectrum. These data further confirm that the effects observed in Fig. 2, c and e, are not due to protein misfolding.