FIGURE 4.
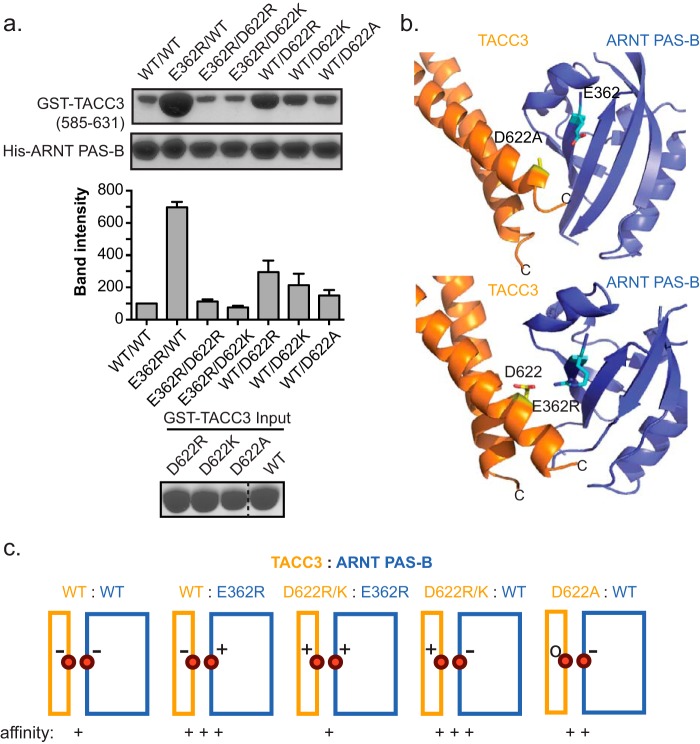
Charge swaps between interacting pairs of residues in ARNT·TACC3 complex further confirms the interacting interface. a, Ni2+ pulldown assay to measure binding between residue pair His-ARNT PAS-B Glu-362 and GST-TACC3 Asp-622 mutants. Residue pairs with favorable (+/−) or neutral (−/0) electrostatics (e.g. E362R/WT, WT/D622R, and WT/D622A) increase the apparent affinity, although repulsive (−/−, +/+) pairs (e.g. E362R/D622R and E362R/D622K) reduce the binding affinity. GST-TACC3 input gel shows equal loading amount. b, crystal structures of ARNT PAS-B·GCN4-TACC3-CT D622A/E629A (upper panel) and ARNT PAS-B E362R·TACC3(585–631) WT (lower panel) show the close proximity of ARNT PAS-B Glu-362 to TACC3 Asp-622 in both structures. The ARNT/TACC3 affinity improvement in both mutant complexes seems likely to be caused by alleviation of the electrostatic repulsion between these negatively charged groups in WT proteins. c, schematic summary of the relationship between interfacial charge-charge interactions and ARNT/TACC3 affinity (TACC3, orange; ARNT PAS-B, blue).