FIGURE 7.
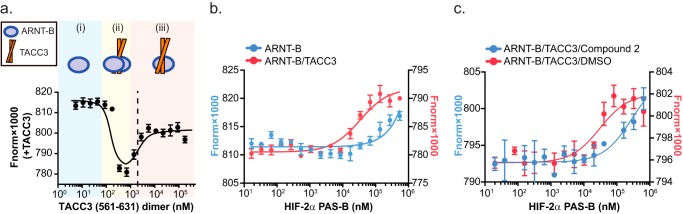
HIF-2α PAS-B cooperatively forms a TACC3-mediated ternary complex with ARNT PAS-B. a, TACC3 binds ARNT PAS-B E362R. TACC3(561–631) dimer was titrated into ARNT PAS-B E362R-fluor and monitored by changes in microscale thermophoresis. With increasing TACC3 concentrations, we observed three binding stages: (i) ARNT PAS-B E362R alone (blue); (ii) two ARNT PAS-B E362R bound to one TACC3 dimer (yellow); (iii) one ARNT PAS-B E362R bound to one TACC3 dimer (red). Subsequent experiments were conducted at 2 μm TACC3 dimer concentration, where a 1:1 ratio of ARNT PAS-B to TACC3 dimer is present (dashed line). b, HIF-2α PAS-B forms a ternary complex with ARNT PAS-B E362R·TACC3 complex. Titrating HIF-2α PAS-B into preformed ARNT PAS-B E362R-fluor·TACC3 complex (red) showed an approximate midpoint of ∼30 μm HIF-2α PAS-B, indicating that HIF-2α PAS-B bound ARNT PAS-B E362R-fluor·TACC3 to form a ternary complex. Titrating HIF-2α PAS-B into ARNT PAS-B E362R-fluor alone (blue) begins to saturate only at higher HIF-2α PAS-B concentrations, consistent with the low affinity between HIF-2α PAS-B and ARNT PAS-B E362R(20). c, HIF-2α PAS-B inhibitor (compound 2 (32)) reduces the apparent affinity of HIF-2α PAS-B to form a ternary complex with ARNT·TACC3. Titrating an equimolar mixture of HIF-2α PAS-B/compound 2 into ARNT PAS-B E362R-fluor·TACC3 showed at a least 10-fold higher apparent Kd value compared with the DMSO control (apparent Kd ∼30 μm), indicating reduced affinity between HIF and ARNT·TACC3 in the presence of compound 2. This result also demonstrated that HIF-2α PAS-B β-sheet is critical for this ternary complex formation.
