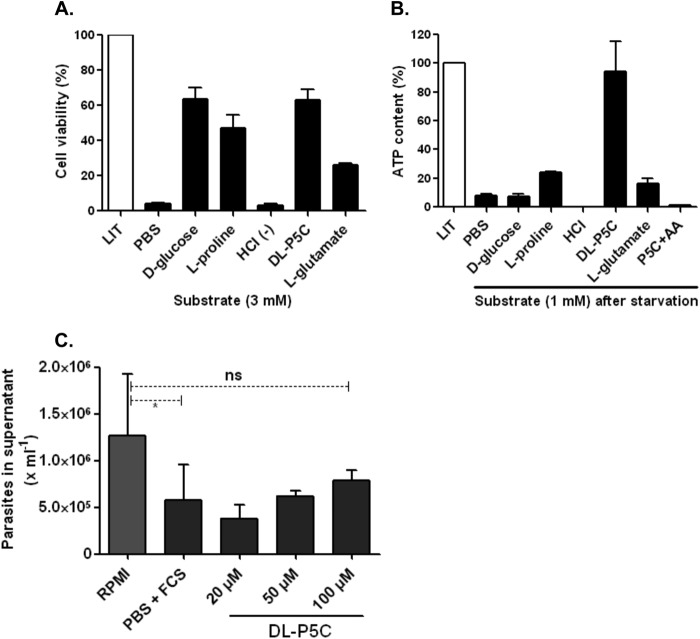
FIGURE 11.
Functional test of P5C in replicative and infective forms of T. cruzi. A, viability test in metabolically stressed cells (over 24 h) in the presence of single nutritional sources (3 mm) as indicated. After the incubation period, the cells were washed once with PBS and incubated (4 h) with MTT reagent in viability assays. Because the P5C purification method was performed in acid medium (HCl), the pH of the medium was adjusted (7.2) with KOH prior to use with the cells. An additional control of P5C-eluting agent (HCl) was also used. Absorbance ratio (590–695 nm) values were converted into percentages of cell viability using LIT as a control. B, determination of ATP levels in epimastigote forms after metabolic starvation. Intracellular ATP was depleted by incubation (30 h at 28 °C) in PBS buffer, followed by a recovery time (1 h) with 1 mm of single catabolic substrates. An additional treatment in the presence of P5C plus 0.5 μm antimycin-A (AA) (a respiratory chain complex IV inhibitor) was also performed. Next, the cells were lysed, and ATP content was determined using a luminescence-based assay following the manufacturer's protocols. The ATP content is expressed relative to cells under normal conditions (as 100%, grown in LIT medium). C, effect of P5C as a catabolic substrate was also tested for the infective TCT forms. iCHO cells were incubated (3 h) under normal (RPMI 1640 medium) and conditional media (PBS + FCS or PBS + P5C), and the number of parasites released into the supernatant (after the 6th day) was determined by hemocytometer counting. No significant (ns) differences were detected when RPMI 1640 medium was compared with PBS + 100 μm P5C, as indicated by one-way analysis of variance followed by Bonferroni's multiple comparison test (*, p < 0.05). Bars in the graph represent the results for three independent replicates (n = 3).