FIGURE 12.
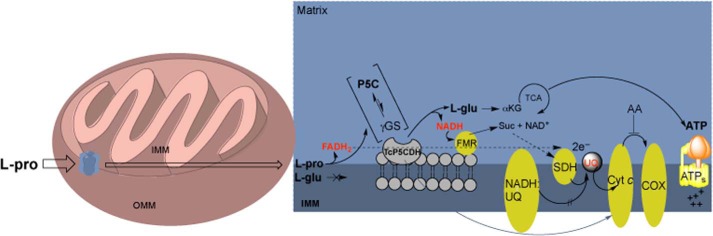
Schematic of the mitochondrial proline metabolic pathway in T. cruzi and its role in bioenergetics. Proline must be taken up from extracellular medium into mitochondria, where it is further oxidized into P5C by FAD-dependent TcProDH concomitantly with the production of FADH2. Next, the P5C is spontaneously converted into γGS, which is enzymatically converted to l-Glu. The TcP5CDH localizes within the inner mitochondrial membrane (IMM) and faces the matrix space, where the conversion of γGS into glutamate occurs at neutral pH. The glutamate produced can be deaminated into α-ketoglutarate (α-KG) and further oxidized in the tricarboxylic acid cycle (TCA), where substrate level phosphorylation can occur at a sufficient succinyl-CoA synthetase level (110). Because the function of the NADH:ubiquinone reductase complex appears limited in T. cruzi (69, 78), NADH can be reoxidized by fumarate reductase (FMR) to produce succinate. Next, e− contained in FADH2 and NADH are transferred (dashed arrows) to the ubiquinone (UQ) pool to a similar degree as succinate dehydrogenase (SDH), promoting the synthesis of ATP by proton F0F1-ATP synthase via the oxidative phosphorylation process. P5C-dependent ATP production is susceptible to inhibition by antimycin-A (AA) at the level of the cytochrome c reductase complex level, thus supporting the above described assumption. However, subsequent protein associations within the mitochondria of this parasite cannot be excluded (gray arrow).
