FIGURE 5.
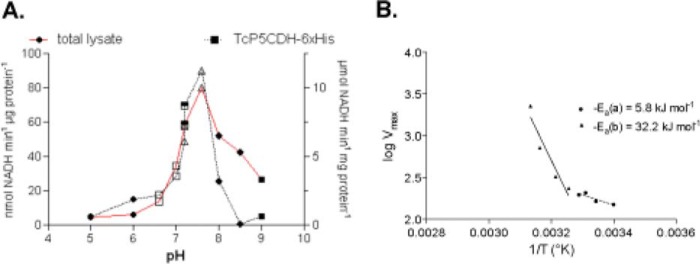
Effect of pH and temperature variations on the TcP5CDH activity. A, pH of the media in the reaction catalyzed by TcP5CDH was modified using different buffer systems. Enzymatic activity was determined in the presence of 2 mm NAD+ disodium salt, 0.3 mm γGS, and 100 mm of reaction buffer as follows: MES-NaOH (pH 5, 6) (filled circles), MOPS-NaOH (pH 6.5, 7) (open squares), HEPES-NaOH, pH 7.2, 7.6 (open triangles), potassium phosphate, pH 7.2, 7.6 (inverted triangles), Tris-HCl, pH 8, 8.5 (diamonds), and CHES (9, 9.5) (filled squares). The reaction was initiated by the addition of the enzyme, and initial velocities were calculated as linear rates at 5 or 15 min (at 30 °C with constant stirring) for the TcP5CDH-His6 or total lysates, respectively. B, effect of temperature variation in reactions catalyzed by TcP5CDH. Enzymatic activity was determined by progressively increasing the reaction temperature (from 20 to 75 °C). Arrhenius plot of the specific activity of TcP5CDH and the temperatures were assayed. y axis, log of Vmax according to temperature values used; x axis, temperature values−1 (Kelvin degrees) tested. The resulting plot was adjusted to a linear function to determine the energy of activation derived from the respective equation (slope = −Ea) (CI = 95%).
