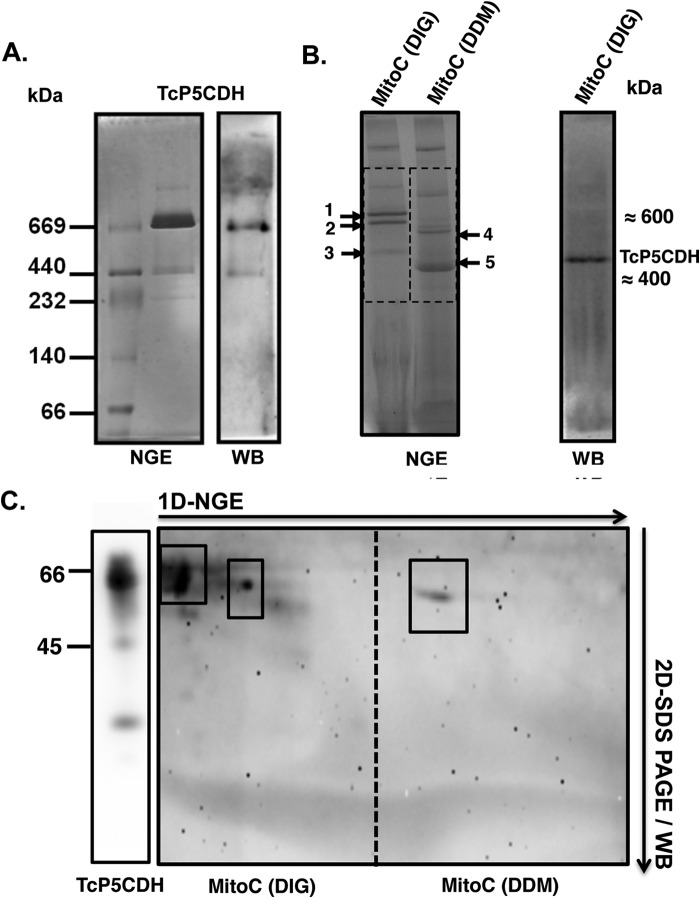
FIGURE 8.
TcP5CDH native gel electrophoretic analysis. A, recombinant TcP5CDH-His6 (fraction P2, Fig. 3A) was resolved by NGE using a 4–16% acrylamide/bisacrylamide gradient gel. Lane 1, molecular mass marker for nondenaturing gels (GE Healthcare); lane 2, recombinant TcP5CDH-His6 (4 μg). The gel was run at 4 °C and further stained with Coomassie Blue R-250 solution as described under “Experimental Procedures.” Right panel, Western blot analysis from the NGE sample using purified anti-TcP5CDH (1:500). B, analysis of the TcP5CDH present in the mitochondrial fractions under native conditions. Samples were prepared from epimastigote forms, and the mitochondrial content (MitoC) was solubilized with either DIG or DDM. The mitochondrial fractions were first resolved by BNGE. Both of the samples (DIG and DDM) were electrotransferred to nitrocellulose membranes and probed with polyclonal anti-TcP5CDH; only DIG samples exhibited immunoreactivity. A single band of high molecular mass (≈450 kDa), presumably mitochondrial membrane-bound, was detected (note: the bands marked 1–5 correspond to the gel areas selected for proteomic analysis by MS/MS as summarized in Table 3). C, two-dimensional (2-D) analysis was performed under denaturing conditions (SDS-PAGE 12%). Samples from the first dimension (1D) native gel were excised (dotted region) and embedded in a gel cast for separation using conventional protein SDS-electrophoresis. After electrophoresis (two-dimensional), the proteins were electrotransferred to nitrocellulose membranes and probed with polyclonal anti-TcP5CDH. The boxes indicate proteins that were distinguished under solubilization conditions, showing two and one reactive bands for the DIG and DDM treatments, respectively. In both cases, the proteins exhibited a molecular mass compatible with that expected for TcP5CDH (∼63 kDa).