FIGURE 1.
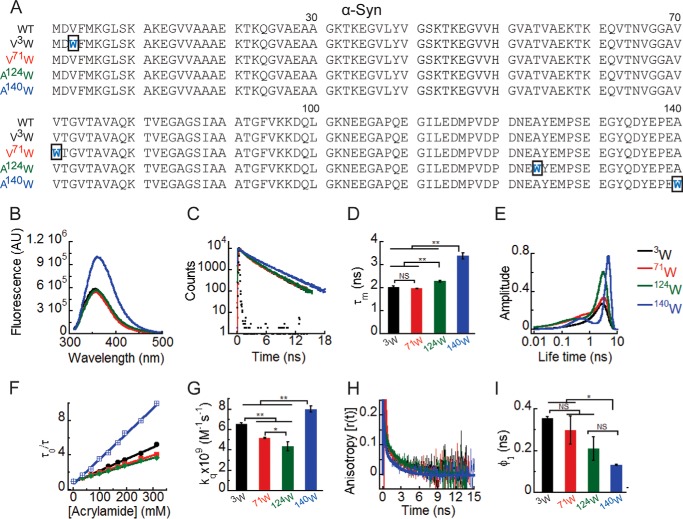
Site-specific structural dynamics of α-Syn. A, amino acid sequences of the protein α-Syn and its four Trp-substituted mutants designed for the study. The substituted Trp residues are shown in blue, and the rectangular boxes indicate the mutation site in the sequences. To delineate the site-specific structural dynamics of α-Syn, freshly prepared low molecular weight solutions of Trp3, Trp71, Trp124, and Trp140 α-Syns were studied using different fluorescence methods. B, steady-state fluorescence spectra showing higher fluorescence intensity of Trp140 compared with Trp3, Trp71, and Trp124. C, time-resolved fluorescence intensity decay kinetics fitted to a three-exponential function (smooth lines) showing slower decay of the fluorescence intensity of Trp140 compared with Trp3, Trp71, and Trp124. D, mean fluorescence lifetime (τm = Σαiτi) values calculated by fitting the fluorescence intensity decays using a three-exponential function represented as a bar diagram (mean ± S.D., n = 3) indicating a different microenvironment experienced by Trp140, Trp124, Trp3, and Trp71. E, fluorescence lifetime distributions obtained by analyzing the time-resolved fluorescence intensity decays using MEM showing that Trp3, Trp71, and Trp124 are conformationally more heterogeneous compared with Trp140. F, Stern-Volmer plots derived from dynamic quenching of fluorescence using lifetime measurements showing a higher slope for Trp140 followed by Trp3, Trp71, and Trp124. G, the bimolecular rate constants for dynamic quenching (kq) calculated from the Stern-Volmer plots and represented as a bar diagram (mean ± S.D., n = 2) indicating that Trp140 is more solvent-exposed followed by Trp3, Trp71, and Trp124. I, time-resolved fluorescence anisotropy decay kinetics fitted to a two-exponential function (smooth lines) showing faster decay of fluorescence anisotropy of Trp140 compared with Trp3, Trp71, and Trp124. J, rotational correlation time associated with the local motion of Trp (φ1) calculated by fitting the fluorescence anisotropy decays and represented as a bar diagram (mean ± S.D., n = 2) indicating that Trp140 is conformationally more flexible compared with Trp3 and Trp71. The statistical significance was calculated by one-way analysis of variance followed by Newman-Keuls multiple comparison post hoc test: *, p < 0.05; **, p < 0.01; NS, not significant, p > 0.05. Error bars represent S.D. AU, arbitrary units.