FIGURE 3.
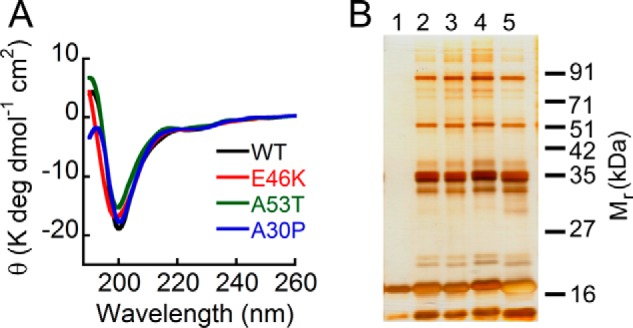
Effect of PD-associated mutations on overall structure and early oligomerization of α-Syn. A, far-UV CD spectra of low molecular weight solutions of WT, E46K, A53T, and A30P α-Syns showing that all the proteins have a minimum in the vicinity of 198–200 nm. B, the early oligomerization was studied by subjecting the freshly prepared low molecular weight solutions of WT α-Syn and its PD-associated mutants to PICUP followed by SDS-PAGE. A silver-stained gel is shown: lane 1, non-cross-linked WT α-Syn; lane 2, cross-linked A30P; lane 3, cross-linked A53T; lane 4, cross-linked E46K; lane 5, cross-linked WT α-Syn. In non-cross-linked WT α-Syn, a band corresponding to monomer (∼17 kDa) was observed, whereas in all cross-linked α-Syns, distinct bands corresponding to monomer (∼17 kDa), dimer (∼35 kDa), trimer (∼51 kDa), and pentamer (∼85 kDa) were observed, indicating that WT α-Syn and its PD-associated mutants have similar early oligomer distributions. deg, degrees.
