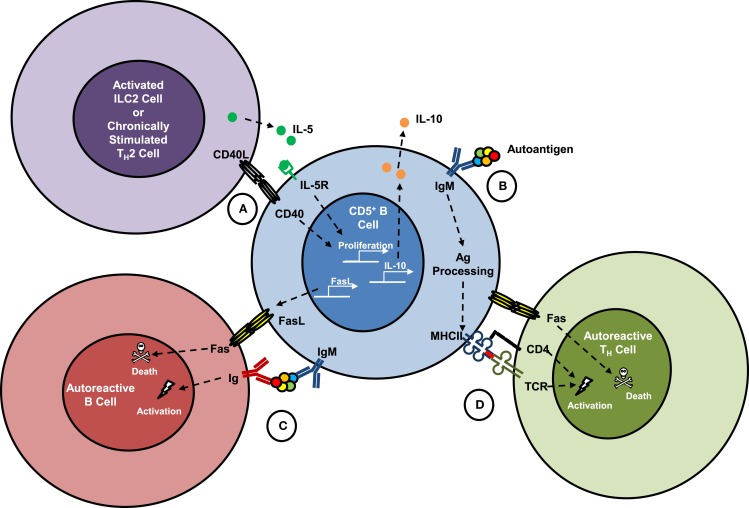
Figure 1.
Hypothesized interactions of killer B cells with other lymphocytes. Fas ligand (FasL) expression is constitutive on mouse spleen and lung CD5+IgMhigh B cells, which have been shown to kill antigen-specific TH cells in vitro. Co-activation of mouse B cells through CD40 and the IL-5 receptor led to proliferation of CD5+ B cells, increased surface expression of FasL and killer function, and production and release of IL-10. (A) Interactions of killer B cells with mucosal type 2 innate lymphoid cells (ILC2), or with chronically stimulated TH2 cells in vivo would be expected to support their growth and functions, but has not been formally proven. (B) Surface immunoglobulins on CD5+ B cells are poly-reactive and are known to recognize autoantigens that once bound can be internalized and processed into peptides, which are then presented to TH cells on class II major histocompatibility (MHCII) molecules. (C) Binding of an autoantigen simultaneously by a killer B cell and an effector B cell could be a mechanism to explain B-cell fratricide, which has been described in several reports. (D) Killer B-cell uptake and presentation of autoantigens to TH cells in the context of FasL–Fas signaling in vivo could lead to activation-induced cell death and is hypothesized to be an important mechanism for maintaining peripheral tolerance and preventing autoimmune diseases. These processes may also play a role in tolerance to food antigens, other allergens, and commensal microbes.