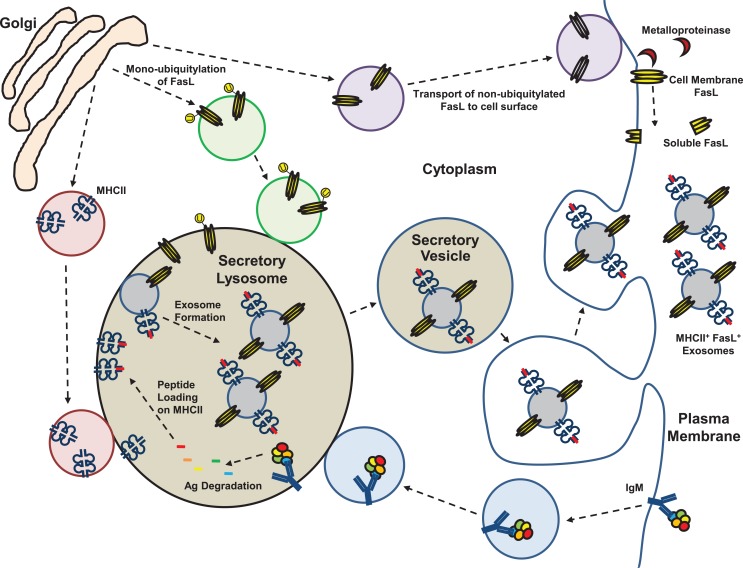
Figure 2.
Membrane, exosome, and soluble forms of Fas ligand. Fas ligand (FasL) protein has been detected in several different forms in mouse and human cells and bodily fluids. Expression of FasL mRNA is generally low and the protein is assembled as a homotrimer in the trans-Golgi prior to transport to the cell surface. Plasma membrane expression of FasL is constitutive on a few specialized epithelial cell types at sites of immune privilege and on CD5+ B cells that have restricted localization. Cell surface expression of FasL is regulated by proteolytic cleavage by metalloproteinases, or by shunting of FasL protein into the secretory lysosomal compartment (SLC). Trafficking of FasL to the SLC is controlled by mono-ubiquitylation. The SLC is a site of antigen processing for molecules transported there from the cell surface by immunoglobulins, and processed peptides are loaded onto MHC class II (MHCII) molecules in the SLC. Through internal budding of the SLC membrane, FasL and MHCII are incorporated into exosomes that are stored in secretory vesicles of killer lymphocytes. Activation of the cell leads to their transport to the cell surface and release into the extracellular space.