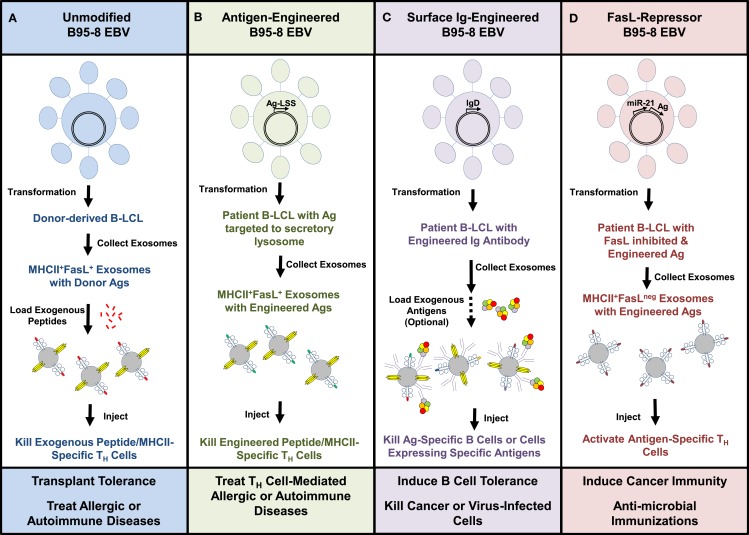
Figure 4.
Potential therapeutic uses of B-LCL exosomes. Exosomes isolated from culture supernatants of EBV-transformed B-LCL were able to kill antigen-specific TH cells in vitro through a FasL-dependent mechanism. This schematic shows ways in which the B95-8 EBV strain might be used in its native form or genetically modified to manufacture customized B-LCL exosomes. (A) Unmodified virus could be used to generate autologous or allogeneic B-LCL that spontaneously produce FasL+MHCII+ exosomes. Replacement of MHC-bound peptides with exogenous peptides could be used to tailor the therapy toward specific antigens. (B) An alternative would be to modify the EBV genome to create B-LCL that express specific antigens linked to a lysosomal sorting sequence (LSS), which would target the proteins to the secretory lysosome where exosomes are assembled. (C) EBV engineered to express rearranged surface immunoglobulins (IgD or IgM) with known specificities onto exosomes may prove useful in targeting antigen-specific B cells, cancer, or virus-infected cells. (D) Inhibition of FasL expression in B-LCL through the repressor miR-21 or other molecules introduced into the EBV genome would be expected to yield FasLneg exosomes that could be used as vaccine adjuvants. Combining these genetic manipulations may further enhance the therapeutic potential of B-LCL-derived exosomes.