Figure 1. Reaction scheme for stable-isotope labeling of cysteines and lysines in proteins.
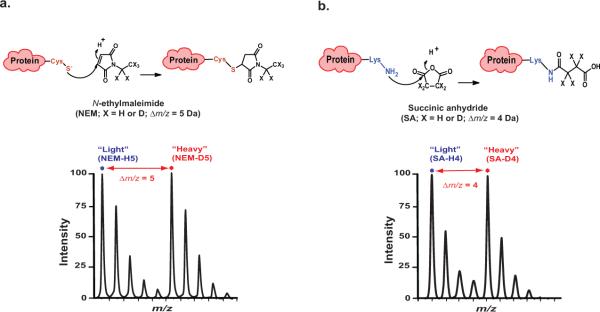
(a) Top, chemical structure of N-ethylmaleimide (NEM-H5 or D5) and the resulting side chain produced by reaction with thiol group of cysteine. Bottom, sample isotope-peak pair (doublet) corresponding to peptide modified at cysteine residue by light and heavy NEM with Δm/z = 5. (b) Top, chemical structure of succinic anhydride (SA-H4 or D4) and the resulting side chain produced by reaction with ε-NH2 group of lysine. Bottom, sample isotope-peak pair (doublet) corresponding to peptide modified at lysine residue by light and heavy SA with Δm/z = 4. Figure adapted from reference55.
