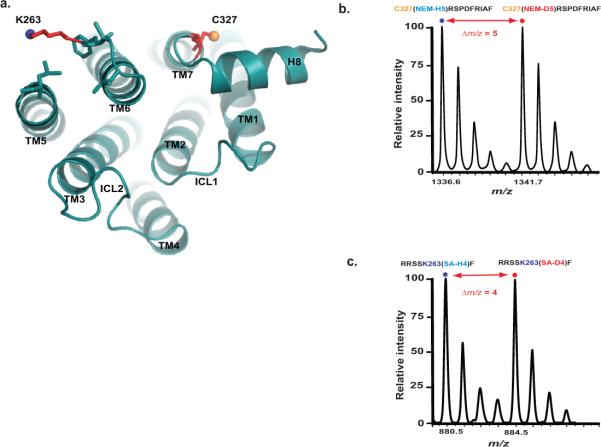
Figure 5. CDSiL-MS based monitoring of conformational rearrangements at critical structural elements of the β2AR.
(a) Cytoplasmic view of β2AR (PDB: 2RH1), showing relative positions of C327 at TM7 (yellow sphere), and K263 (blue sphere) at TM6. (b) Singly charged ion ([M+H]+) isotope-peak pair corresponding to a chymotryptic peptide (327CRSPDFRIAF336) modified at C327 by a light and heavy NEM (m/z 1336.6 and 1341.7, respectively; Δm/z = 5). (c) Singly charged ion ([M+H]+) isotope-peak pair corresponding to a chymotryptic peptide (259RRSSKF264) modified at K263 by light and heavy succinic anhydride (m/z 880.5 and 884.5, respectively; Δm/z = 4).