A method for highly efficient human induced pluripotent stem (iPS) cell derivation from adult blood under clinically compliant conditions is reported. The revised episomal vectors and the removal of animal-sourced materials resulted in feeder-free and xeno-free iPS cell generation. Pooled cultures were purified by the presence of the TRA-1-60 pluripotency surface antigen to reduce clonal variations and for repaid iPS cell expansion. These new improvements permit to generate clinically compliant iPS cell lines for therapeutic applications.
Keywords: Human iPS cells, Reprogramming, Human blood mononuclear cells, Plasmid expression, Episomal vectors, Feeder-free, Xeno-free cell culture
Abstract
Reprogramming human adult blood mononuclear cells (MNCs) cells by transient plasmid expression is becoming increasingly popular as an attractive method for generating induced pluripotent stem (iPS) cells without the genomic alteration caused by genome-inserting vectors. However, its efficiency is relatively low with adult MNCs compared with cord blood MNCs and other fetal cells and is highly variable among different adult individuals. We report highly efficient iPS cell derivation under clinically compliant conditions via three major improvements. First, we revised a combination of three EBNA1/OriP episomal vectors expressing five transgenes, which increased reprogramming efficiency by ≥10–50-fold from our previous vectors. Second, human recombinant vitronectin proteins were used as cell culture substrates, alleviating the need for feeder cells or animal-sourced proteins. Finally, we eliminated the previously critical step of manually picking individual iPS cell clones by pooling newly emerged iPS cell colonies. Pooled cultures were then purified based on the presence of the TRA-1-60 pluripotency surface antigen, resulting in the ability to rapidly expand iPS cells for subsequent applications. These new improvements permit a consistent and reliable method to generate human iPS cells with minimal clonal variations from blood MNCs, including previously difficult samples such as those from patients with paroxysmal nocturnal hemoglobinuria. In addition, this method of efficiently generating iPS cells under feeder-free and xeno-free conditions allows for the establishment of clinically compliant iPS cell lines for future therapeutic applications.
Introduction
Derivation of human induced pluripotent stem (iPS) cells was first described in 2007 using human fibroblasts [1, 2]. The original protocol using genome-inserting viral vectors expressing Oct4, Sox2, Klf4, and c-Myc transgenes (or similar combinations) has proved to be successful in many cell types, including hematopoietic cells [3–15]. Compared with human fibroblasts, which must be established in culture from biopsies of adult donors, mononuclear cells (MNCs) from umbilical cord blood (CB) or peripheral blood (PB) can be obtained from existing blood stocks or freshly drawn samples. Furthermore, these hematopoietic MNCs can be also expanded quickly to a proliferating cell population that is critical to efficient iPS cell derivation. For most iPS applications, it is better not to use T or B lymphocytes that have pre-existing DNA rearrangements at the V(D)J locus and other regions in the human genome, although they are more abundant in PB MNCs and easier to expand in culture [6–15]. For the same reason, it is highly desirable to make human iPS cells without the use of viral vectors or other genome-inserting vectors that alter the genome, allowing for faithful disease modeling or safer downstream applications of cell therapies in patient-derived iPS cells [15]. Although others have used a combination of four Sendai viral vectors to generate integration-free human iPS cells reprogrammed from hematopoietic cells [13, 14], we have focused on using nonviral vectors to generate human iPS cells from blood MNCs that could be more applicable to generating clinical-grade iPS cell lines.
Since 2011, several publications have demonstrated that episomal vectors are capable of reprogramming human blood MNCs to integration-free iPS cells [16–22]. We, and several other groups, have focused on using either hematopoietic progenitors (expressing the CD34 surface antigen) or myeloid-erythroid cells, both lacking V(D)J rearrangements such as found in committed T and B cells. Although CD34+ hematopoietic progenitor cells are highly proliferative and ready for efficient reprogramming after 2–5 days culture, they are rare in adult PB (<0.01%) unless the donors have been treated with a stem cell mobilization regimen. Most of the MNCs in adult PB are lymphocytes (∼50%), although hematopoietic progenitor cells at various developmental stages also exist, including myeloid-erythroid restricted progenitor cells. We have reported using a culture condition that selectively supports the formation and expansion of erythroblasts for subsequent iPS derivation that is now widely used [16, 18]. Although a decline in cell numbers in the first 5–6 days (likely owing to cell death of lymphocytes or mature myeloid cells) was observed, we obtained a near homogenous population of proliferating erythroid cells by days 8–12 in the selective culture of PB MNCs and ∼2 days sooner with CB MNCs [16, 18]. Therefore, we can easily establish such a proliferating cell population of mainly erythroblasts for reprogramming from unfractionated MNCs from PB, CB, or bone marrow aspirates, without previous selection of the rare CD34+ cells. The efficiencies of iPS cell derivation by episomal vectors delivered by a single round of nucleofection into culture-expanded CD34+ cells and erythroblasts are similar [16, 18].
Several studies observed that the efficiency of iPS cell formation from newborn CB-derived erythroblasts is much higher than that from adult PB erythroblasts [16–20, 23]. The addition of a second episomal vector expressing SV40 large T antigen (pEB-Tg) significantly enhanced the efficiency of deriving human iPS cells from adult PB MNCs by the pEB-C5 episomal vector (expressing Oct4, Sox2, Klf4, c-Myc, and Lin28 genes) that alone is sufficient to reprogram CB cells [16]. The derived human iPS cells are highly similar to human embryonic stem (ES) cells in phenotype and function and do not have vector insertion or overt alterations in the genome [16, 18, 23]. The efficiency from adult PB MNCs, however, is still 20–50-fold lower than that from CB MNCs and is donor cell dependent [16, 18, 23]. For example, for one adult donor, we might obtain dozens or even hundreds of iPS cell clones from 2 million culture-expanded erythroblasts; however, from a different adult donor MNC sample, only a few iPS cell clones might be formed using the same method and vector preparation. This prompted us to improve the episomal vectors and culture conditions to achieve a highly consistent, efficient, and robust method to establish human iPS cells from adult blood MNCs.
By incorporating recent advances, we have established a method to achieve consistent and efficient generation of human iPS cells from adult PB MNCs using a combination of three EBNA1/OriP episomal vectors. Furthermore, we have eliminated the need for mouse feeder cells and animal-sourced proteins used in current culture systems of human iPS cell derivation, especially from blood cells. Finally, we have simplified the reprogramming procedure by eliminating the conventional practice of manually picking individual iPS cell clones. Instead, we pooled dozens to hundreds of primary iPS cell clones and quickly expanded the iPS cells to sufficient numbers for subsequent use. The pooling method also helps to avoid or reduce the genetic variations among the different selected iPS clones from the same donor. These improvements permit a consistent and reliable method to generate human iPS cells from a large and diverse population and to generate iPS cells efficiently and under the clinically compliant conditions required for future therapeutic applications.
Materials and Methods
Improved Episomal Vectors for Reprogramming Human Blood MNCs
We used the pCEP4 plasmid backbone containing the EBNA1/OriP replicon for making improved nonintegrating reprogramming vectors, such as in our previous study [16]. First, we deleted and replaced the DNA fragment (from NruI to NheI, ∼2.7 kb) containing the hygromycin selection gene controlled by the herpes simplex virus type 1 (HSV-1) thymidine kinase (TK) promoter and polyadenylation DNA sequences and the cytomegalovirus (CMV) promoter, with a short linker restoring the NruI and NheI sites. Next, we inserted the spleen focus-forming virus (SFFV) long terminal repeat promoter/enhancer (∼400 bp), which showed better gene expression than the CMV promoter in human hematopoietic cells [20, 22, 24]. The DNA fragment of the SFFV promoter/enhancer was synthesized by GenScript (Piscataway, NJ, http://www.genscript.com), based on the DNA sequence of the pSFFV-neo-BCL-XL expression vector [24]. Third, the woodchuck post-transcriptional regulatory element, widely used to enhance mRNA stability and/or translational efficiency [5, 25], was synthesized and cloned into the modified pCEP4 vector downstream to the reprogramming genes, as previously described [20]. Next, cDNAs of the various reprogramming genes (OCT4, SOX2, KLF4, and c-MYC) were inserted into the modified (M) episomal vectors expressing a single transcript of two linked cDNAs. One, called MOS (modified vector expressing OCT4 and SOX2), expresses both human OCT4 and SOX2 proteins linked by a 2A self-cleavage peptide. The second one, called MMK (modified vector expressing c-MYC and KLF4), expresses human c-MYC and KLF4 proteins, also linked by a 2A element. The third, MBX (modified vector expressing BCL-XL), expresses BCL-XL alone. The configuration of these vectors is similar to the three vectors previously published [22]. We also made a different episomal vector, called GBX (modified vector expressing GFP and BCL-XL), expressing green fluorescent protein (GFP) and human BCL-XL, linked by a P2A self-cleavage peptide, using a piggyBac (PB)-based plasmid (provided by Dr. H. Bai) in the laboratory of Dr. Z. Wang of Johns Hopkins University. Illustrative plasmid maps are shown in supplemental online Figure 1. The detailed plasmid maps and DNA sequences of vectors will be deposited to Addgene.org and available to academic investigators.
The episomal vectors pEB-C5 and pEB-Tg (expressing SV40 large T antigen) have previously been described in detail [16, 18]. They are available to academic investigators via Addgene.org (as plasmids 28213 and 28220). The three episomal vectors of the Okita-Yamanaka combination [21] were also obtained from Addgene.org.
Isolation and Culture of PB MNCs Under a Serum-Free Condition
The Johns Hopkins University and Institute of Hematology of the Chinese Academy of Medical Sciences institutional review boards approved the use of anonymous or deidentified human blood samples from consented adult donors and patients. In addition, the Johns Hopkins University Institutional Stem Cell Research Oversight Committee and the institutional authority in the People’s Republic of China, in accordance with local regulations, approved the laboratory research on the derivation and use of human iPS cell lines.
MNCs were isolated from adult PB using the standard procedures either by loading 35 ml of diluted blood onto a layer of 15 ml of Ficoll-Paque Premium (GE Healthcare Life Sciences, Pittsburgh, PA, http://www.gelifesciences.com), in a 50-ml conical tube [18] or Vacutainer tubes with an existing Ficoll layer (catalog no. 362761; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). The MNCs above the Ficoll layer were harvested and washed and subjected to lysis of the contaminated red blood cells [18]. The washed MNCs were either frozen (ideally 10 million per vial) or cultured immediately to establish erythroblast cultures using a serum-free medium (SFM) [16, 18]. In brief, the SFM consisted of 50% Iscove’s modified Dulbecco’s medium and 50% Ham’s F12 medium, synthetic lipids, and insulin-transferrin-selenium supplement (all from Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com), 5 mg/ml or 0.5% bovine serum albumin (BSA), 50 μg/ml of L-ascorbic acid (2-phosphate sesquimagnesium salt), 200 μM 1-thioglycerol, 2 mM glutamine (all from Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), supplemented with human stem cell factor (SCF) (255-SC, 50 ng/ml; R&D Systems, Minneapolis, MN, http://www.rndsystems.com), interleukin (IL)-3 (10 ng/ml; product code 200-03, PeproTech, Rocky Hill, NJ, http://www.peprotech.com), human holo-transferrin (100 µg/ml; product code 2914-HT, R&D Systems), erythropoietin (2 U/ml; National Drug Code [NDC] 59676-303-01, Procrit [epoetin alfa]), insulin-like growth factor 1 (40 ng/ml; product code 100-11, Peprotech), and dexamethasone (1 μM; Sigma-Aldrich). For MNCs expanded under a myeloid-favoring condition, the cell were cultured in the SFM with SCF (50 ng/ml) plus FLT3 ligand (50 ng/ml), IL-3 (10 ng/ml), and IL-6 (10 ng/ml) for 8–12 days before reprogramming. In more recent experiments, we replaced 0.5% BSA with the Food and Drug Administration (FDA)-approved Plasbumin-25 (NDC 13533-684-20; Grifols, Barcelona, Spain, http://www.grifols.com), which is made from pooled human venous plasma and contains 25% human albumin, at the same final concentration as the BSA. The erythroblast cultures (typically after 8–12 days) were used for reprogramming by nucleofection of episomal vectors.
Expansion of Human iPS Cells in the E8 Medium on Vitronectin as Adhesion Substrates
Mouse embryonic fibroblasts (MEFs) were either purchased from GlobalStem (Gaithersburg, MD, http://www.globalstem.com) or made in house from 13.5-day-old embryos of the CF-1 mouse strain [5, 7, 9, 16, 18, 26]. For feeder-free cultures, established human iPS cell lines were expanded using the E8 medium (catalog no. A1517001; commercialized by Life Technologies), on either Matrigel (1:30; BD Biosciences) or vitronectin (0.5 μg/cm2; catalog no. A14701SA; Life Technologies), as described previously [27, 28]. The E6 medium (catalog no. A1516401; Life Technologies) that contained the same components as E8 except for basic fibroblast growth factor (bFGF) (100 ng/ml) and transforming growth factor-β1 (TGFβ1; 2 ng/ml) was used as the basal medium for comparison with the E8 medium to test the effects of TGFβ1 (2 ng/ml). The cells were maintained in an undifferentiated state with the E8 medium on vitronectin and routinely passaged (∼1:10 every 3 days) as either small clumps using the EDTA method or single cells after enzymatic digestion by Accutase (catalog no. A6964; Sigma-Aldrich), as previously described [28]. To enhance single cell survival, a ROCK inhibitor Y27632 (final concentration 10 μM; Stemgent, Cambridge, MA, http://www.stemgent.com; or other sources) was added to the medium for the first 24 hours after seeding [28].
Conventional Culture Condition of Human iPS Cell Derivation for Testing New Episomal Vectors
To test new EBNA1/OriP episomal vectors compared with the pEB-C5 and pEB-Tg combination or Okita-Yamanaka combination, 2 × 106 cultured erythroblasts were mixed with a total of 10 μg of plasmid DNA of episomal vectors for nucleofection (Lonza, Walkersville, MD, http://www.lonza.com). We used 4 μg of MOS, 4 μg of MMK, and 2 μg of MBX (or GBX). When the Okita-Yamanaka vector combination was used, we followed the original report [21]. We either used Nucleofector II (Lonza), with the CD34 cell solution (Nucleofector Kit, catalog no. VPA-1003; Lonza) and T016 setting, as previously described [16, 18], or the newer 4D Nucleofector System (Lonza), with the P3 solution and the human CD34+ cell (EO-100) setting.
After nucleofection, the treated cells were immediately cultured in the same erythroblast medium for 2 more days, as previously described for our conventional protocol [16, 18]. On day 2, the nucleofected cells were plated into 3 wells (in 12-well plates) coated with irradiated MEFs and cultured in MEF medium (Dulbecco’s modified Eagle’s medium [DMEM] plus 10% fetal bovine serum [FBS]). The next day, 50% of the medium was exchanged with the ES cell medium with sodium butyrate (NaB; 0.25 mM) that consistently enhanced human iPS cell derivation under various culture conditions [16, 18, 26]. The human ES medium includes 20% knockout serum replacement containing BSA and other proprietary components (Life Technologies) and other supplements, as previously described [16, 18]. The straight human ES medium was added daily, as needed, for the next 7 days. Then, MEF-conditioned medium (CM) collected with the ES medium and mixed with an equal volume of fresh ES medium was added daily for the next 5–6 days to provide trophic factors and sustain the iPS cell derivation process.
Improved and Simplified Culture Conditions of Human iPS Cell Derivation
To provide a simpler, feeder-free and xeno-free culture condition and to improve reprogram efficiency, we gradually replaced FBS and BSA with recombinant or human sourced proteins. Stepwise, we replaced 10% FBS used in the MEF medium with 0.5% human albumin (from Plasbumin) and bone morphogenetic protein 4 (BMP4; 5 ng/ml). This medium was added at day 2 after nucleofection to culture hematopoietic cells on vitronectin (0.5 μg/cm2) or Matrigel (1:30 dilution of the BD Biosciences stock). From day 3, 50% of the medium was exchanged with the E8 medium, together with NaB (0.25 mM), as the only other supplement.
Numeration and Isolation of Reprogrammed iPS Cells Based on the TRA-1-60 Surface Antigen
By day 14 (after nucleofection), we performed live staining of the adherent culture (in 3 wells) of reprogrammed cells for each sample, using the TRA-1-60 monoclonal antibody (catalog no. MAB4360; EMD Millipore, Billerica, MA, http://www.emdmillipore.com), as previously described in detail [18, 26]. After numeration of the TRA-1-60+ (and TRA-1-60−) colonies, we harvested all the reprogrammed cells in bulk using Accutase (Sigma-Aldrich). The harvested cells were plated into 2–3 wells of a 6-well plate coated with vitronectin and cultured in the E8 medium with the ROCK inhibitor Y27632 (10 μM). After 1 passage (within 3 or 4 days), expanded human iPS cell cultures were harvested by Accutase digestion to make single cell suspensions and labeled with the anti-TRA-1-60 antibody (conjugated with R-phycoerythrin [PE]). Then, the cells expressing a high level of TRA-1-60 cell surface antigen were enriched (>95%) with anti-PE magnetic microbeads, using the MACS Technology and a mini-MACS column (catalog no. 130-095-816; Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com).
Flow Cytometric Analysis for Expression of GFP Marker and Cell Surface Antigens
Cell aliquots (≤1 million) were washed in phosphate-buffered saline (PBS) and resuspended in 100 μl of PBS and then stained with the appropriate dilution of the antibody and incubated for 30 minutes at room temperature (RT) in the dark. The antibodies used were CD59-PE (catalog no. MHCD5904; Life Technologies) and alkaline phosphatase (1:100; catalog no. NB600-540; Novus Biologicals, Littleton, CO, http://www.novusbio.com). For TRA-1-60 staining, primary antibody TRA-1-60 (1:200; MAB4360 mouse IgM; EMD Millipore) and Alexa Fluor 555-conjugated anti-mouse IgM secondary antibody (1:500; Life Technologies) were diluted in PBS and added in combination.
Immunocytochemistry Staining
Human iPS cell cultures were washed 3 times in PBS and fixed in freshly prepared 4% paraformaldehyde in PBS (pH 7.4) for 20 minutes. For staining nuclear antigens, such as NANOG, the fixed cells were also permeabilized in 0.1% Triton X-100. The cells were washed 3 times and stained with primary antibody against NANOG (1:100; catalog no. 500-P236; Peprotech) at RT for 2 hours. The cells were washed 3 times and incubated for 2 hours with secondary antibody, Alexa Fluor 488 goat anti-rabbit (1:500; catalog no. A-31628; Life Technologies) at RT. After the washes, the cells were stained with CD59-PE (1:20; catalog no. MHCD5904; Life Technologies) at RT for 2 hours. The other antibodies used included OCT4 (1:50; catalog no. sc-9081; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) and SSEA4 (1:300; catalog no. MC-813-70; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, http://www.dshb.biology/uiowa.edu).
Pluripotency Assays for Newly Derived iPS Cells by New Methods
Formation of embryoid bodies (EBs) and teratoma was used just as in vitro and in vivo assays for pluripotency, as previously described [16, 18]. Similarly, karyotyping of human iPS cells was conducted by the previously described method [16, 18].
Results
Improved Episomal Vectors for Highly Efficient and Consistent Reprogramming of Human Blood MNCs
From the previous method using episomal vectors and one-time nucleofection of human hematopoietic cells [16, 18, 20, 22], we incorporated the recent advancements and devised improved vectors for efficient reprogramming of adult hematopoietic cells (supplemental online Fig. 1). First, we deleted the hygromycin selection gene (under the control of the HSV-1 TK promoter) from the commonly used pCEP4 backbone episomal vector. Next, we split multiple reprogramming genes in 3 episomal vectors, each expressing 1–2 genes at maximal levels, just as in most related studies [17, 19–22, 29]. Increasing evidence has indicated that the dose of EBNA1 gene expression is critical to reprogramming efficiency. Higher efficiencies have been achieved by either cotransfecting EBNA1 mRNA [27] or cotransfection of an additional episomal vector that expresses the EBNA1 gene simultaneously [21]. Therefore, the use of the third EBNA1/OriP episomal vector (which also expresses another critical reprogramming transgene) effectively increases the level of EBNA1 gene expression. The 3 separate plasmids also provide the flexibility to use different ratios or doses of various reprogramming genes (a total of 5–7 transgenes). We tested whether a third EBNA1/OriP plasmid (expressing additional genes) could enhance reprogramming of human erythroblasts to iPS cells under our culture conditions. The BCL2L1 gene encoding BCL-XL was tested first, inspired by the results from several recent studies. We had previously observed that somatic cells expressing a high level of the BCL-2 gene (highly related to BCL2L1) tended to have higher efficiency of human iPS derivation [16] and that cotransfection of a BCL-2 expression vector increased iPS derivation efficiency [23]. In addition, a recent study found that BCL-XL enhances single-cell survival and expansion of human ES cells [30]. More directly, a recent report demonstrated that adding an episomal vector expressing the BCL2L1 gene significantly upregulated (up to 10-fold) the reprogramming efficiency by the 4 Yamanaka factors of cultured human myeloid cells from adult PB MNCs [22].
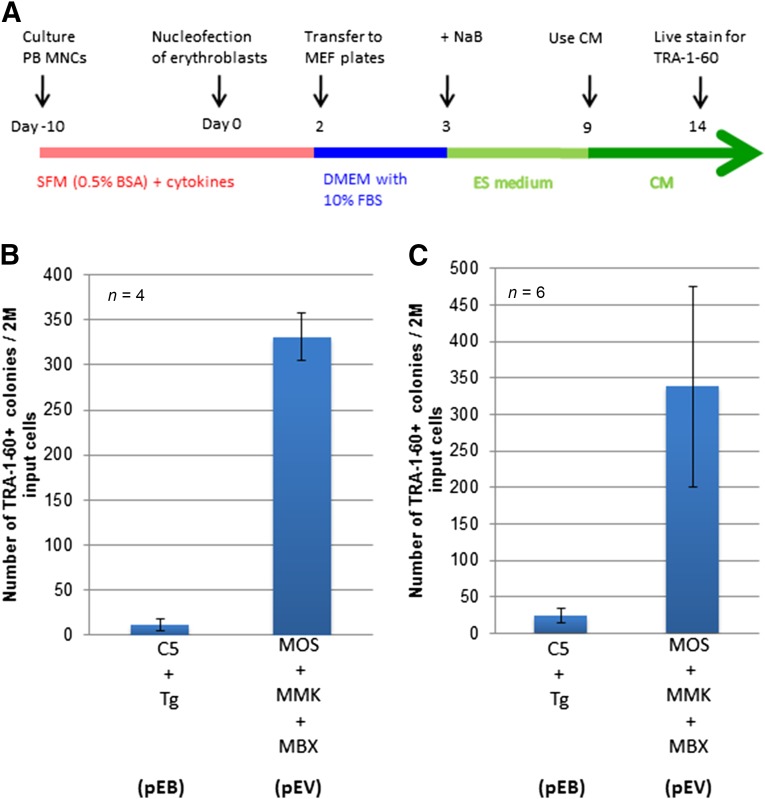
Using the strategy described in the previous paragraph, we used the three episomal vectors based on the modified and shortened pCEP4 vector to express OCT4/SOX2 (MOS), MYC/KLF4 (MMK), and BCL-XL (MBX) under the SFFV promoter (supplemental online Fig. 1). The combination of the three vectors (MOS, MKM, and MBX, also called the pEV combination) was chosen to reprogram human cultured erythroblasts that were expanded from adult PB MNCs (Fig. 1), in direct comparison with the previously tested combination of two EBNA1/OriP vectors, pEB-C5 and pEB-Tg (the pEB combination) [16]. Two million culture-expanded erythroblasts were nucleofected once with the same optimal amount of DNA and then cultured under the equivalent culture conditions with MEF feeders [16, 18] (Fig. 1A). In the first series of experiments conducted with erythroblasts from four healthy donors (Fig. 1B), many more TRA-1-60+ iPS cell clones were established using the pEV combination than using the pEB combination (pEB-C5 and pEB-Tg). In the second series of experiments conducted with expanded erythroblasts from MNCs of six adult donors, we observed similar results (Fig. 1C). Although variations in iPS derivation efficiencies exist among different adult donors, the reprogramming efficiencies using the pEV vector combination were consistently higher (10–50-fold) compared with the pEB combination. We also compared the pEV combination with the Okita-Yamanaka combination of three episomal plasmids [21]. The results showed the pEV combination had significantly higher efficiencies with all three different adult MNC samples we tested (supplemental online Fig. 2).
Figure 1.
BCL-XL functions in synergy with MOS/MMK to confer efficient induced pluripotent stem cell derivation of adult PB MNCs. (A): Schematic diagram of PB MNC expansion and reprogramming by C5/Tg (pEB) or MOS/MMK/MBX (pEV) using the standard culture condition. (B, C): Comparison of PB MNC reprogramming by the pEB and pEV combination in two series of experiments conducted in Baltimore and Tianjin, respectively. The differences between the pEB and pEV vectors were statistically significant (p < .01) in both sets of experiments. Abbreviations: BSA, bovine serum albumin; CM, conditioned medium with MEF; DMEM, Dulbecco’s modified Eagle’s medium; ES, (human) embryonic stem (cells); FBS, fetal bovine serum; M, million; MBX, modified vector expressing BCL-XL; MEF, mouse embryonic fibroblast; MMK, modified vector expressing c-MYC and KLF4; MNCs, mononuclear cells; MOS, modified vector expressing OCT4 and SOX2; NaB, sodium butyrate; PB, peripheral blood; SFM, serum-free medium;Tg, SV40 large T antigen.
We also tested whether MNCs expanded under a culture condition favoring myeloid cell proliferation could be more efficient in generating a proliferating cell population and/or in reprogramming expanded hematopoietic cells compared with the erythroblast culture condition. We used MNCs from two different adult donors, stimulated them with one of two culture media, and reprogrammed expanded cells using the same pEV vector combination. We found that the cell number decline was similar in the first 6 days under both culture conditions (supplemental online Fig. 3). However, the cells in the erythroblast culture condition started to proliferate after day 6 and by day 10 had led to a net cell gain. In contrast, the cell numbers in the myeloid culture condition did not increase (supplemental online Fig. 3A). The myeloid culture condition also resulted in a mixed cell population (containing lymphoid cells in addition to CD33+ myeloid cells) among the cells surviving at day 8, and the erythroblast culture by day 8 showed a much more homogenous cell population (supplemental online Fig. 3B). Reprogramming efficiencies were also determined later using TRA-1-60+ colony counting. We found the erythroblast culture condition to be more suitable for expanding PB MNCs for effective reprogramming (supplemental online Fig. 3C). Therefore, we selected the pEV episomal vectors, including MOS and MMK, in combination with a BCL-XL expression vector, and the erythroblast culture condition for the subsequent experiments.
Successful Reprogramming of Adult PB MNCs From Patients
We have used the pEV combination to reprogram human erythroblast samples from various donors and patients, including a patient diagnosed with paroxysmal nocturnal hemoglobinuria (PNH). PNH results from an acquired mutation that abolishes or reduces the function of phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIG-A) in hematopoietic stem cells. PIG-A is essential to the formation of glycosylphosphatidylinositol (GPI)-anchored proteins, and patients with PNH lack all GPI-anchored proteins such as CD59 of the hematopoietic lineage. Previously, we successfully knocked out the PIG-A gene in human ES and iPS cells [31]. We demonstrated that GPI-anchored proteins such as CD59 and alkaline phosphatase (ALPase) are not essential to the growth of undifferentiated ES and iPS cells in culture but are required for differentiation to mesodermal cell types [31, 32]. However, using 2 million erythroblasts that we managed to expand from the PB MNCs of patients with PNH, the previous pEB vector combination generated very few or no iPS cell colonies.
When we used the pEV combination to reprogram erythroblasts derived from PB MNCs from a patient with PNH, we observed dozens of iPS cell colonies expressing the TRA-1-60 surface antigen after 14 days (supplemental online Fig. 4). The individual TRA-1-60+ colonies were manually picked and expanded, as previously described [18, 26]. These colonies displayed human iPS cell morphology and phenotype identical to the wild type control iPS cells after expanding in the E8 medium (supplemental online Fig. 4A). Although both wild type and PNH iPS cells expressed a pluripotency marker NANOG in the nuclei, the PNH iPS cell colonies lacked the expression of CD59, a GPI-anchored protein missing in PNH hematopoietic cells. We further confirmed the absence of CD59 by flow cytometric analysis with single cell suspensions (supplemental online Fig. 4B). Overall, although both the wild type and PNH iPS cells express the TRA-1-60+ surface antigen, only the wild type iPS cells expressed CD59 and ALPase. The PNH-T3-6 clone completely lacked the GPI-anchored proteins ALPase and CD59 on cell surface (supplemental online Fig. 4A, 4B).
Use of a GFP-Containing BCL-XL Vector to Monitor Reprogramming at Multiple Steps
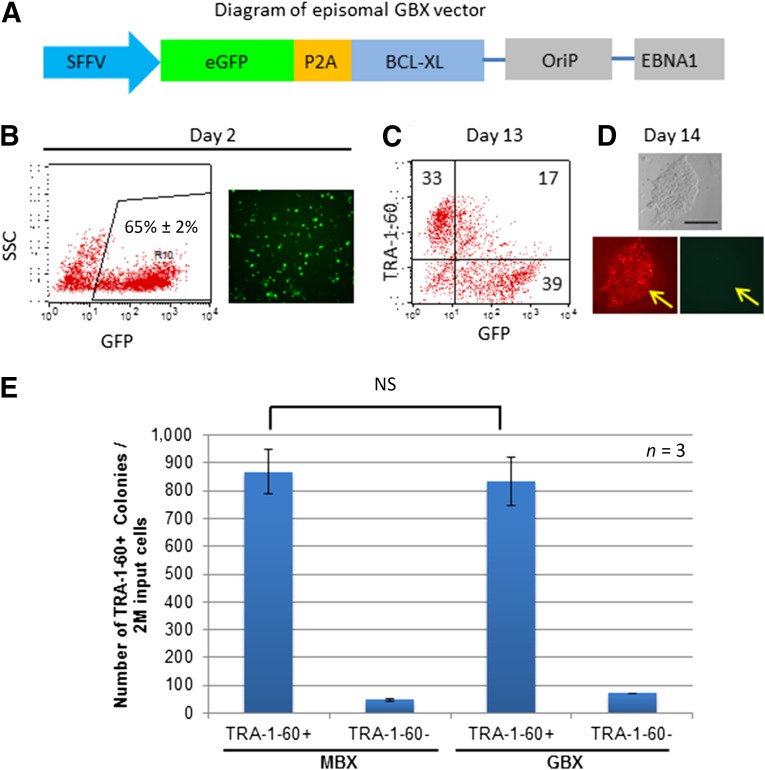
Having confirmed the importance of adding the BCL-XL episomal vector, we made an alternative BCL-XL vector expressing the enhanced (e)GFP protein in frame with the P2A self-cleavage peptide and BCL-XL protein downstream to eGFP (Fig. 2A). This vector (called GBX) is particularly useful in monitoring the reprogramming process, which lasts approximately 2–3 weeks. After transfection of the GBX episomal vector, together with the 2 other vectors (MOS and MMK) into erythroblasts, GFP expression was detected after 2 days, allowing for estimation of the transfection efficiency (∼65% of erythroblasts survived; Fig. 2B). During the reprogramming process, we observed a reduction in GFP-expressing cells, especially in the cells acquiring the TRA-1-60 antigen. By day 14, most TRA-1-60+ colonies were no longer positive for GFP (Fig. 2C, 2D), which aided in selection of fully reprogrammed iPS cell colonies that silenced transgene expression. We confirmed that, when used in combination with the MOS and MMK episomal vectors (the pEV combination), the bicistronic GBX has an efficiency in generating TRA-1-60+ iPS cell colonies similar to that of the MBX vector (Fig. 2E).
Figure 2.
Introduction of the GFP reporter to the BCL-XL vector helped to monitor nucleofection efficiency and identify fully reprogrammed clones. (A): Diagram of newly constructed GBX vector. (B): GFP expression was used to monitor nucleofection efficiency at day 2 after nucleofection. The microphotograph was taken at 10× magnification. (C): Reprogrammed cells were analyzed at day 13 using flow cytometric analysis. Many cells that gained TRA-1-60 tended to lose GFP expression. (D): Imaging analysis showed that fully reprogrammed iPS-like colonies that gained TRA-1-60 expression (stained in red) lacked the GFP signal (arrow). Scale bar = 200 μm. (E): The GFP-expressing GBX plasmid has potency similar to that of the MBX plasmid expressing BCL-XL alone in reprogramming adult PB MNCs in combination with two other episomal vectors (MOS and MMK). Abbreviations: EBNA1, Epstein-Barr nuclear antigen 1; eGFP, enhanced green fluorescent protein; GBX, modified vector expressing GFP and BCL-XL; GFP, green fluorescent protein; iPS, induced pluripotent stem cells; M, million; MBX, modified vector expressing BCL-XL; MNCs, mononuclear cells; NS, no significant differences on statistical analysis; PB, peripheral blood; SFFV, spleen focus-forming virus; SSC, side scatter.
Although inclusion of the GFP-expressing episomal vector might complicate the process of generating clinically compliant iPS cells (even if present in reprogrammed cells only transiently), the GBX vector greatly improved our ability to generate iPS cells consistently and reliably from hundreds of donors.
Elimination of Mouse Feeder Cells and Conditioned Medium
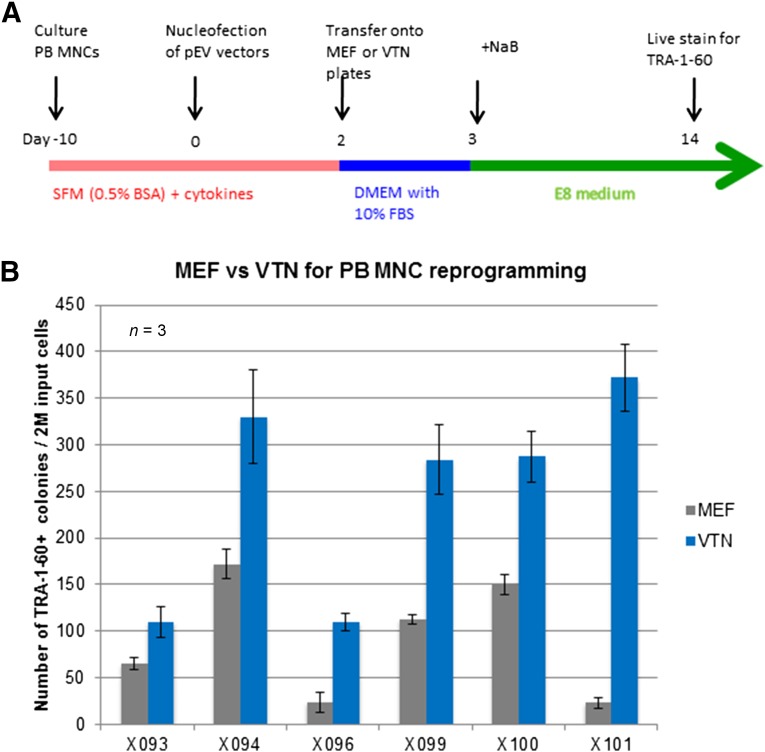
In our previously published protocol, we used MEF feeders and their conditioned medium for reprogramming human adult blood cells to achieve the highest efficiency with the previous episomal vector combination [16, 18]. Based on the recent success of using the simplified (and xeno-free) E8 medium and vitronectin as the coating substrate to expand human iPS and ES cells [27, 28], we replaced both the ES medium starting at day 3 and the subsequent MEF CM by the E8 medium throughout (Fig. 3A). The derived iPS cells that were manually picked on day 14 could also be expanded in the E8 medium on vitronectin-coated tissue plates, as we have shown with previously established human iPS cells [28].
Figure 3.
Replacement of MEFs by vitronectin as a cell culture substrate maintained high reprogramming efficiency with the improved vector combination. (A): Schematic diagram of using VTN to substitute for MEFs as the coating material for tissue plates used at day 2 after nucleofection. A chemically defined, xeno-free E8 medium was used to replace the human ES cell medium that has animal products in the serum replacement. (B): VTN was shown to sustain high reprogramming efficiency under the MOS/MMK/MBX (pEV) vector combination with six different samples of adult PB MNCs of randomly selected donors. Abbreviations: BSA, bovine serum albumin; DMEM, Dulbecco’s modified Eagle’s medium; ES, embryonic stem (cell); FBS, fetal bovine serum; M, million; MEF, mouse embryonic fibroblast; MNCs, mononuclear cells; NaB, sodium butyrate; PB, peripheral blood; SFM, serum-free medium; VTN, vitronectin.
Encouraged by these results, we attempted to completely replace the MEFs and CM with vitronectin and the E8 medium during the reprogramming process. Instead of plating on MEF-coated plates at day 2 after nucleofection, the cells were plated on vitronectin-coated plates (Fig. 3A) but still using the same MEF medium containing 10% FBS from days 2 to 3 (Fig. 3A). After a side-by-side comparison with cultured erythroblasts from 6 different PB MNC donors, we discovered that vitronectin and the E8 medium (added at day 3) had higher efficiencies in forming human iPS cell colonies compared with the MEFs we used (Fig. 3B). In the early stage of our effort to replace MEFs, we also tested Matrigel (BD Biosciences), an extracellular matrix mixture derived from mouse tumors, as a replacement for MEFs. However, it showed even greater variations among the different batches, even with the same donor cells (data not shown). Therefore, we chose vitronectin as a coating substrate for a feeder-free condition to reprogram human PB MNCs to adherent iPS cells.
TGFβ1 is one component in the E8 medium that can support human iPS cell proliferation. However, it has been suggested that TGFβ1 inhibits the mesenchymal-to-epithelial transition that was a part of the reprogramming process in several studies [33]. Some studies also demonstrated that adding small molecule inhibitors such as SB431542, which blocks TGFβ1, and activin signaling further enhanced the efficiency of iPS derivation from mouse and human fibroblasts [34]. To assess whether eliminating TGFβ1 from the E8 medium would further increase the efficiencies of reprogramming from blood cells, we used the E6 medium (lacking both TGFβ1 and bFGF), added the necessary bFGF (therefore, TGFβ1−), and compared that with the E8 medium (TGFβ1+). We found that the E8 medium consistently generated more iPS cell colonies than the E6 medium with bFGF (supplemental online Fig. 5). This finding implies the presence of TGFβ1 in the E8 medium does not have any negative effect on reprogramming from human blood MNCs. For convenience, we used the E8 medium from day 4 after nucleofection and throughout the reprogramming and expansion processes.
Efficient Expansion of Pooled iPS Cell Clones After Reprogramming
The conventional method of establishing iPS cell lines is to manually pick individual colonies with favorable morphology and/or antigen staining emerging (14 days after nucleofection of hematopoietic cells in our system). For example, we used the TRA-1-60 monoclonal antibody and performed live staining of the reprogrammed cultures when large human iPS-like (or ES-like) colonies were observed [9, 16, 18]. The fully reprogrammed cells were recognized by the TRA-1-60 antibody (Fig. 2D) and individually picked for subsequent expansion. This step was critical in the early stage of the human iPS cell derivation method because many partially reprogrammed (TRA-1-60−) cells were also present that proliferated more efficiently than fully reprogrammed TRA-1-60+ iPS cells. Manual separation of the best individual iPS cell colonies from the bulk culture became the standard practice in the past few years, despite several shortcomings.
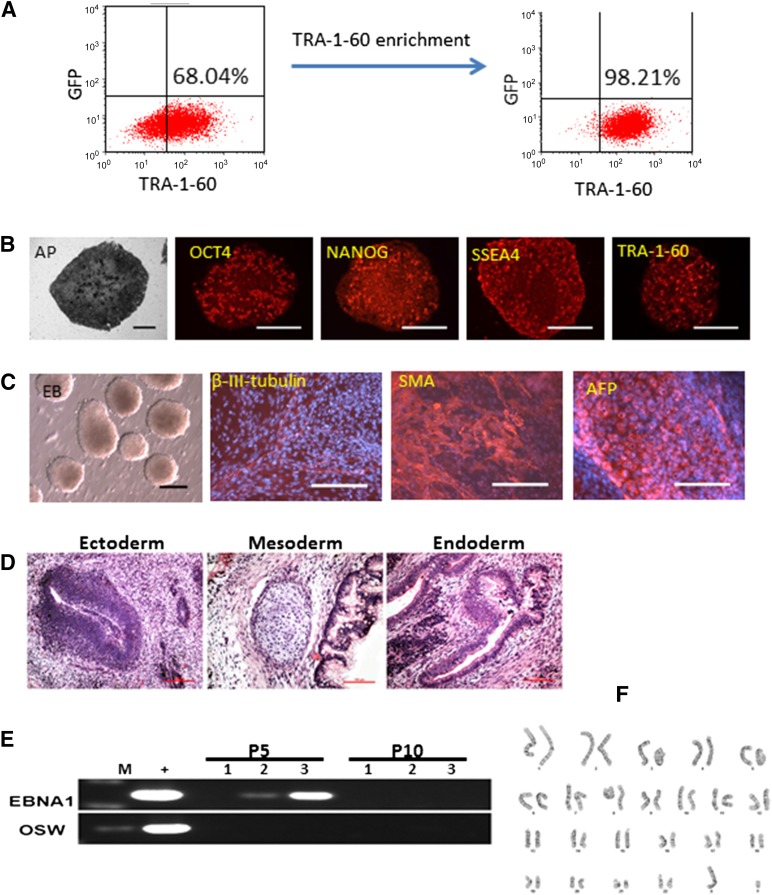
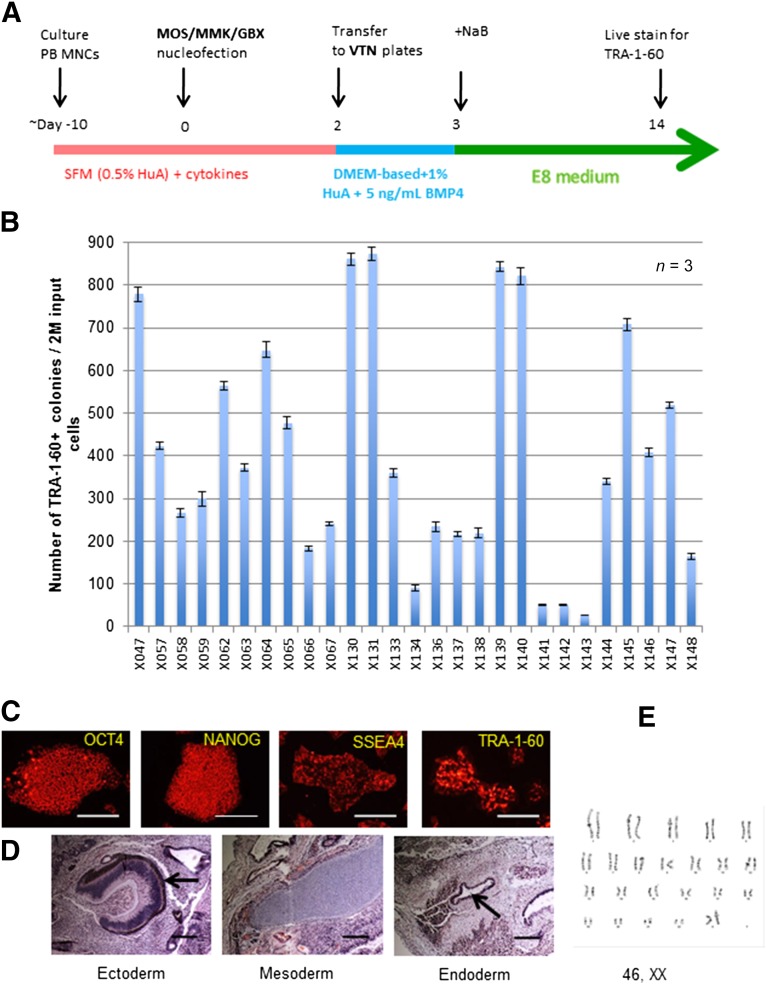
In our improved reprogramming system with episomal vectors and culture conditions that appear to suppress the growth of partially reprogrammed cells, we typically observed dozens to hundreds of TRA-1-60+ colonies at day 14 and very few TRA-1-60− colonies. We can also expand the numbers of TRA-1-60+ cells (and colonies) by harvesting all the cells at day 14 and replating them under the same culture conditions with the E8 medium and vitronectin. The large numbers of TRA-1-60+ cells present in culture after one passage allowed us to purify TRA-1-60+ cells by MACS using a mini-size column (Fig. 4A). The cells that expressed a high level of the TRA-1-60 cell surface antigen were selectively purified and were cultured at a higher cell density (5,000–10,000 cells per cm2 in 24-well [2 cm2] or even 6-well [∼10 cm2] plates) than that we could afford when an individually picked iPS cell colony (100–1,000 cells per well in 24-well plates) was cultured and expanded. The pooled and purified iPS cells were expanded efficiently; in fact, we can make frozen stocks at passage 2 (p2) and have more cells for characterization at early passages (p3–p5). Similar to the individually picked iPS clones that fulfill the criteria for pluripotent stem cells, the pooled and purified iPS cell population expressed pluripotency markers (Fig. 4B), formed EBs containing various cell types derived from all three germ layers (Fig. 4C), and formed teratoma as the evidence of their pluripotency in vivo (Fig. 4D). We also examined the presence of episomal vector DNA in the 3 different pooled iPS cell lines after 5 and 10 passages (Fig. 4E). Using 2 sets of PCR primers that can specifically amplify the MOS vector sequence, we detected the presence of the vector sequence in 2 of 3 iPS cell lines examined at passage 5. However, the vector DNA was no longer detectable after 10 passages, confirming the transient nature of the episomal vectors in these pooled iPS cells. We confirmed that the pooled iPS cell cultures maintained a normal karyotype after 15 passages (Fig. 4F), just as in the vast majority of clonally picked and expanded iPS cells derived by episomal vectors and under the improved culture conditions.
Figure 4.
Enrichment of TRA-1-60 expression cell population and characterization of induced pluripotent stem (iPS) cells generated by the improved vector combination and culture condition. (A): Use of microbeads and MACS to enrich emerging human iPS cells that express a high level of TRA-1-60 cell surface antigen. (B): Staining for pluripotency markers on a representative iPS cell line (X117, derived using the MOS+MMK+GBX vector combination). (C): Pluripotency test in vitro by embryoid body (EB) formation. EB-mediated differentiation in suspension culture in 10 days was followed by an additional 2-day adherent cell culture. Cells expressing 3 germ layer markers were found. Scale bars = 200 μm. (D): In vivo pluripotency test via teratoma formation. Cells derived from endoderm, mesoderm, and ectoderm germ layers were found. (E): Polymerase chain reaction-based detection of vector-specific DNA sequences (EBNA1 and OSW) in derived and expanded iPS cells. Three representative iPS cell lines were randomly selected and analyzed at passages 5 and 10. Vector DNA was not found in the expanded iPS cells after 10 passages. (F): A normal karyotype of representative iPS cells from donor X003 (46, XY), at passage 15 of expansion. Abbreviations: AFP, α-fetoprotein; AP, alkaline phosphatase; EBNA1, Epstein-Barr nuclear antigen 1; GFP, green fluorescent protein; M, marker (DNA ladder); P, passage; SMA, smooth muscle actin.
Eliminating Animal-Derived Proteins in Defined Culture Medium
We next attempted to replace the FBS and BSA used in the day 2 postnucleofection culture medium and in the medium used to establish erythroblast cultures with either defined recombinant proteins or FDA-approved, clinical-grade, human-derived biologics (Fig. 5). We found that the 0.5% BSA used in human erythroblast culture can be substituted with the 0.5% human albumin present in FDA-approved Plasbumin-25, made from human plasma and containing 25% human albumin (data not shown). The 10% FBS used in the MEF medium appears to be critical for hematopoietic cells to adhere to vitronectin when plated in culture at day 2 after nucleofection. We found that adding diluted Plasbumin (final medium containing 0.5% human albumin) into DMEM in place of 10% FBS helped cell survival and resulted in iPS cell generation; however, the reprogramming efficiency was reduced (supplemental online Fig. 6). We next tested whether adding a growth factor such as recombinant human BMP4, which is known to be present in serum and affect cell fates, could complement human albumin and increase the reprogramming efficiency [35, 36]. We found that pleiotropic BMP4 added at day 2 after nucleofection resulted in more adherent nucleofected (GFP+) cells at day 4 (supplemental online Fig. 6A). More importantly, BMP4-treated cells (from days 2 to 4) showed better reprogramming efficiency than those when human albumin was used alone (supplemental online Fig. 6B). Although the MEF medium containing 10% FBS (Fig. 2A; supplemental online Fig. 6A) used from days 2 to 3 showed the best results, it was no longer essential when improved vectors and culture conditions (including the use of human albumin and recombinant BMP4) were used.
Figure 5.
Reprogramming PB MNCs in a serum-free and xeno-free cell culture condition. (A): A schematic diagram to highlight the use of human albumin to replace 0.5% bovine serum albumin in SFM and 10% fetal bovine serum in the medium used between days 2 and 3. (B): Improved vector combination and the xeno-free reprogramming protocol showed consistently high reprogramming efficiency in 27 samples. (C): Pluripotency marker staining of induced pluripotent stem (iPS) cells derived under xeno- and feeder-free conditions from donor X057. Scale bars = 200 μm. (D): In vivo pluripotency test via teratoma formation. Cells derived from endoderm, mesoderm, and ectoderm germ layers were found. Scale bars = 200 μm. (E): A normal karyotype of expanded iPS cells from donor X057 (46, XX), at passage 15. Abbreviations: BMP4, bone morphogenetic protein 4; DMEM, Dulbecco’s modified Eagle’s medium; GBX, modified vector expressing GFP and BCL-XL; HuA, human albumin; MMK, modified vector expressing c-MYC and KLF4; MNCs, mononuclear cells; MOS, modified vector expressing OCT4 and SOX2; NaB, sodium butyrate; PB, peripheral blood; SFM, serum-free medium; VTN, vitronectin.
In synergy with the pEV vectors and the improved feeder-free reprogramming culture conditions (using the E8 medium and vitronectin as substrates), we successfully reprogrammed erythroblasts from >200 adult PB MNC samples; 27 are shown in Figure 5B. The numbers of iPS (TRA-1-60+) colonies from 2 million nucleofected erythroblasts ranged from 20 to 880 (Fig. 5B); however, we established iPS cells for every donor sample using the pooling method. After TRA-1-60+ cell purification and additional expansion, human iPS cells expressed pluripotency markers such as OCT4, NANOG, and SSEA4, as well as TRA-1-60, shown in the iPS cells derived from subject X057 at passage 10 (Fig. 5C). They can form teratoma with three germ layer structures (Fig. 5D), an in vivo assay of pluripotency. X057 iPS cells also maintained a normal karyotype when examined at passage 15 (Fig. 5E). Thus, we established a reliable and facile method to consistently achieve human iPS cell derivation from adult PB MNCs in a completely feeder-free and xeno-free manner.
Discussion
We report the development of a facile and highly efficient method to derive human iPS cells from blood MNCs under clinically compliant culture conditions (Fig. 6). The improved combination of 3 episomal vectors increased the reprogramming efficiency of adult PB MNCs by 10–50-fold over those we previously used (Fig. 1). The improved efficiency also allowed us to derive iPS cells from PB MNCs of patients with PNH, which was unsuccessful with a previous episomal vector combination [16, 18]. We also compared the pEV episomal vectors with the combination of 3 episomal vectors expressing OCT4, SOX2, KLF4, L-MYC, LIN28, and a small hairpin RNA against p53 that was developed by Okita et al. [21, 37] to reprogram erythroblasts from adult PB MNCs. Our data suggest that the pEV vector combination is more efficient than the Okita-Yamanka vector combination [21, 37] in reprogramming human erythroblasts. However, the pEV episomal vectors were optimized to reprogram human hematopoietic cells and might not be adequate for reprogramming other cell types, such as fibroblasts and keratinocytes that were previously used by others.
Figure 6.
A facile procedure for generating footprint-free human iPS cell lines from blood MNCs under a xeno-free and defined condition. Abbreviation: iPS, induced pluripotent stem.
Human blood MNCs have become increasingly used for integration-free iPS cell derivation owing to its easy sampling and in vitro expansion [16, 21, 22]. We also showed that the erythroblast culture condition is effective for expanding proliferating erythroblasts from several milliliters of adult human PB MNCs; a healthy person would have ∼2–10 million MNCs from 1 ml of PB blood. To date, among the more than 200 subjects we have tested, we have easily achieved at least 2 million proliferating erythroblasts from each sample for reprogramming. No additional cell purification (e.g., CD34+ cell isolation or lymphocyte depletion) is necessary before or after erythroblast culture. We routinely used nucleofection to deliver plasmid DNA to 2 million erythroblasts, although starting with fewer cells (0.5–1 million) is also feasible for most erythroblast samples. However, one drawback of this method is that a relatively larger starting cell number (0.5–2 million) is required for consistent and reliable iPS cell derivation compared with methods using Sendai viruses or mRNA transfection. A better and gentler nucleofection technique with higher transfection efficiency would definitely help reduce the starting cell number. Overall, our method allowed consistent and reliable derivation of human iPS cells from >200 adult PB erythroblast samples tested (as of December 2014).
Recombinant Sendai viruses were also shown to be able to efficiently reprogram human hematopoietic cells, including nonlymphocytes to integration-free iPS cells [13, 14, 38, 39]. It is convenient to use premade Sendai viruses (e.g., CytoTune; Life Technologies) from a commercial vendor for reprogramming both hematopoietic and nonhematopoietic cells for laboratory research. However, no current good manufacturing practice (cGMP)-grade Sendai virus kit is yet available, and the current kit is limited to research use only [40]. Transient transfection of several mRNAs for human cell reprogramming is another integration-free method with unique advantages [40]. However, owing to rapid mRNA degradation, multiple deliveries of mRNA (up to 12–14 times daily) to target cells are usually required [41]. To date, no successful reprogramming from blood cells has been achieved using the mRNA approach, in the presence or absence of microRNAs [40]. In addition, the increased workload makes the mRNA reprogramming protocol less facile than that of making and using episomal vectors. A recent report showed that synthetic self-replicative RNA can reprogram human newborn and adult fibroblast and reduced the transfection number to just once [42] (Simplicon; EMD Millipore). However, RNA transfection is more immunogenic than DNA delivery; thus, the study heavily relied on an interferon inhibitor B18R through cotransfection that made the protocol more complicated [42]. Whether the synthetic self-replicative RNA can generate human iPS cells from blood MNCs remains to be determined.
Several studies have been conducted with the aim of making cGMP-quality iPS cell lines [43, 44]; however, the complicated processes of vector production and using animal-derived proteins and/or infectious viruses for cell culture will be inherently challenging to make cGMP-level iPS cells under such conditions. Compared with the production of viral vectors or even mRNAs, cGMP-grade plasmid DNA can be easily manufactured in large quantity and stored for a long time, which provides a great benefit to derive iPS cells under cGMP conditions. We also tried to eliminate all the animal-derived, undefined proteins such as FBS, albumin preparations, and MEFs, which often have batch-to-batch variations and do not comply with cGMP requirements. In the present study, we found that MEFs and conditioned medium can be replaced with vitronectin and the E8 medium. In addition, we used clinical-grade, cGMP-compliant human albumin to replace the FBS used in the culture medium during day 2–3 culture after nucleofection and the BSA used in the erythroblast expansion medium (Fig. 3A). We found human clinical-grade albumin (Plasbumin) can successfully substitute for these animal-derived products and support iPS cell generation. Although Plasbumin is obtained and processed from large pools of adult human plasma, which has not been chemically defined and is subject to batch-to-batch variations, we obtained more consistent reprogramming efficiencies using several different batches of Plasbumin than using various batches of BSA. In the future, we will test whether recombinant human albumin can replace Plasbumin to further reduce the potential batch-to-batch variation and possible contamination by infectious agents. Recently, a cGMP-quality P3 electroporation solution was developed [45]. Using a cGMP-certified 4D nucleofection device, we will be able to conduct the entire procedure of human iPS cell derivation from blood MNCs under cGMP-compliant conditions.
In the past several years, many investigators realized that the manual picking of individual iPS cell colonies increasingly became a hurdle to the efficient derivation and selection of iPS cells that are best representative of the genome of the parental somatic cells. The manual picking procedure, in addition to being time-consuming, difficult to standardize, and highly subjective, also has a scientific limitation. Increasing evidence has demonstrated that the iPS cells derived from individual colonies, which are likely from a single somatic cell (i.e., clonal), captures all the genetic variants of that cell. Additional genetic and epigenetic variations, either random or selective, can be gained during the 40–50 rounds of cell division required for iPS cell derivation and expansion. Whole genome sequencing of iPS genomic DNA compared with DNA of the parental somatic cells found that one iPS cell clone can differ from another iPS clone from the same experiment in ∼1,000 single nucleotides [46, 47]. Although this is a relatively small number (3 × 109 nucleotides in a haploid human genome), it confounds the ability to select representative individual iPS cells for genetic or biological studies. In the present study, we pooled reprogrammed culture and used a MACS mini-column to purify iPS cells according to the presence of the TRA-1-60 surface antigen. The purified cells from dozens to hundreds of primary iPS cell clones from each reprogrammed sample can be quickly expanded in sufficient numbers for subsequent applications. Other groups have also started to perform similar selection by pooling iPS cells derived from human fibroblasts [48, 49]. Both studies showed that high-quality iPS cells can be obtained from pooling of bulk cultures and expanded more efficiently than using individual colonies. A frozen stock can be made as early as passage 2. If genetic mutations are found in the expanded iPS cells in later passages, the frozen cells will be in sufficient numbers to be expanded quickly for various applications.
Conclusion
We have developed a facile method to efficiently generate iPS cells under feeder-free and xeno-free conditions. This method will allow us to develop a procedure for establishing clinically compliant iPS cell lines for future therapeutic applications.
Supplementary Material
Acknowledgments
We thank Dr. Hao Bai for providing the BCL-XL-P2A-eGFP piggyBac plasmid, Dr. Xiao-Bing Zhang for information and advice on constructing the improved pEV vectors, and Drs. Zack Wang and Evan Braunstein for discussions and critical reading. This work was supported in part from the Maryland Stem Cell Research Fund (Grant 2011-MSCRF-0088) and NIH (Grants U01 HL107446 and 2R01 HL-073781) to L.C., the National Basic Research Program of China (Grant 2011CB964800) and National Natural Science Foundation of China (Grant 81421002) to T.C., and the National Natural Science Foundation of China (Grant 81400152) to H.G. L.C. is also supported by the Edythe Harris Lucas and Clara Lucas Lynn Chair in Hematology at Johns Hopkins University School of Medicine.
Author Contributions
B.-K.C. and H.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.G.: conception and design, collection and/or assembly of data, data analysis and interpretation; S.N.D., Y.W., and Y.L.: collection and/or assembly of data, data analysis and interpretation; J.S.: provision of study material or patients; Z.Y.: data analysis and interpretation, manuscript writing; T.C.: financial support, manuscript writing, final approval of manuscript; L.C.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 4.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 5.Mali P, Ye Z, Hommond HH, et al. Improved efficiency and pace of generating induced pluripotent stem cells from human adult and fetal fibroblasts. Stem Cells. 2008;26:1998–2005. doi: 10.1634/stemcells.2008-0346. [DOI] [PubMed] [Google Scholar]
- 6.Loh YH, Agarwal S, Park IH, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–5479. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Z, Zhan H, Mali P, et al. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgetti A, Montserrat N, Aasen T, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mali P, Chou BK, Yen J, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28:713–720. doi: 10.1002/stem.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Lee MR, Hangoc G, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou J, Sweeney CL, Chou BK, et al. Oxidase-deficient neutrophils from X-linked chronic granulomatous disease iPS cells: Functional correction by zinc finger nuclease-mediated safe harbor targeting. Blood. 2011;117:5561–5572. doi: 10.1182/blood-2010-12-328161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merling RK, Sweeney CL, Choi U, et al. Transgene-free iPSCs generated from small volume peripheral blood nonmobilized CD34+ cells. Blood. 2013;121:e98–e107. doi: 10.1182/blood-2012-03-420273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye L, Muench MO, Fusaki N, et al. Blood cell-derived induced pluripotent stem cells free of reprogramming factors generated by Sendai viral vectors. Stem Cells Translational Medicine. 2013;2:558–566. doi: 10.5966/sctm.2013-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan HK, Toh CX, Ma D, et al. Human finger-prick induced pluripotent stem cells facilitate the development of stem cell banking. Stem Cells Translational Medicine. 2014;3:586–598. doi: 10.5966/sctm.2013-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu K. All roads lead to induced pluripotent stem cells: the technologies of iPSC generation. Stem Cells Dev. 2014;23:1285–1300. doi: 10.1089/scd.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu K, Yu J, Suknuntha K, et al. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowey SN, Huang X, Chou BK, et al. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012;7:2013–2021. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Chau KF, Vodyanik MA, et al. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 2011;6:e17557. doi: 10.1371/journal.pone.0017557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng X, Neises A, Su RJ, et al. Efficient reprogramming of human cord blood CD34+ cells into induced pluripotent stem cells with OCT4 and SOX2 alone. Mol Ther. 2012;20:408–416. doi: 10.1038/mt.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okita K, Yamakawa T, Matsumura Y, et al. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 22.Su RJ, Baylink DJ, Neises A, et al. Efficient generation of integration-free IPS cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS One. 2013;8:e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mali P. Engineering Human Cells [PhD thesis]. Baltimore, MD: Johns Hopkins University; 2011.
- 24.Chao DT, Linette GP, Boise LH, et al. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lois C, Hong EJ, Pease S, et al. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 26.Mali P, Ye Z, Chou BK, et al. An improved method for generating and identifying human induced pluripotent stem cells. Methods Mol Biol. 2010;636:191–205. doi: 10.1007/978-1-60761-691-7_12. [DOI] [PubMed] [Google Scholar]
- 27.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Chou BK, Dowey S, et al. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res (Amst) 2013;11:1103–1116. doi: 10.1016/j.scr.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang QE, Liu SP, Liu YF, et al. [Efficient reprogramming of human cord blood CD34(+) cells for formation of induced pluripotent stem cells with non-integrating plasmid system] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2013;21:728–734. doi: 10.7534/j.issn.1009-2137.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Bai H, Chen K, Gao YX, et al. Bcl-xL enhances single-cell survival and expansion of human embryonic stem cells without affecting self-renewal. Stem Cell Res (Amst) 2012;8:26–37. doi: 10.1016/j.scr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou J, Maeder ML, Mali P, et al. Gene targeting of a disease-related gene in human induced pluripotent stem cells and embryonic stem cells. Cell Stem Cell. 2009;5:97–110. doi: 10.1016/j.stem.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan X, Braunstein E, Ye Z, et al. Generation of GPI anchor protein deficient blood cells from human induced pluripotent stem cells. Stem Cells Translational Medicine. 2013;2:819–829. doi: 10.5966/sctm.2013-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 34.Lin T, Ambasudhan R, Yuan X, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–808. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera B, Inman GJ. A rapid and sensitive bioassay for the simultaneous measurement of multiple bone morphogenetic proteins. Identification and quantification of BMP4, BMP6 and BMP9 in bovine and human serum. BMC Cell Biol. 2009;10:20. doi: 10.1186/1471-2121-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu RH, Peck RM, Li DS, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 37.Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 38.Trokovic R, Weltner J, Nishimura K, et al. Advanced feeder-free generation of induced pluripotent stem cells directly from blood cells. Stem Cells Translational Medicine. 2014;3:1402–1409. doi: 10.5966/sctm.2014-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett R, Ornelas L, Yeager N, et al. Reliable generation of induced pluripotent stem cells from human lymphoblastoid cell lines. Stem Cells Translational Medicine. 2014;3:1429–1434. doi: 10.5966/sctm.2014-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlaeger TM, Daheron L, Brickler TR, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33:58–63. doi: 10.1038/nbt.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshioka N, Gros E, Li HR, et al. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell. 2013;13:246–254. doi: 10.1016/j.stem.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awe JP, Lee PC, Ramathal C, et al. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. doi: 10.1186/scrt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durruthy-Durruthy J, Briggs SF, Awe J, et al. Rapid and efficient conversion of integration-free human induced pluripotent stem cells to GMP-grade culture conditions. PLoS One. 2014;9:e94231. doi: 10.1371/journal.pone.0094231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lonza. cGMP Cell Line NucleofectorTM Kits. Available at http://www.lonza.com/products-services/bio-research/transfection/nucleofector-kits-for-cell-lines/cgmp-cell-line-nucleofector-kits.aspx. Accessed February 15, 2015.
- 46.Cheng L, Hansen NF, Zhao L, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by non-integrating plasmid expression. Cell Stem Cell. 2012;10:337–344. doi: 10.1016/j.stem.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: Potential causes and implications for application. Cell Stem Cell. 2013;13:149–159. doi: 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willmann CA, Hemeda H, Pieper LA, et al. To clone or not to clone? Induced pluripotent stem cells can be generated in bulk culture. PLoS One. 2013;8:e65324. doi: 10.1371/journal.pone.0065324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valamehr B, Abujarour R, Robinson M, et al. A novel platform to enable the high-throughput derivation and characterization of feeder-free human iPSCs. Sci Rep. 2012;2:213. doi: 10.1038/srep00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.