Abstract
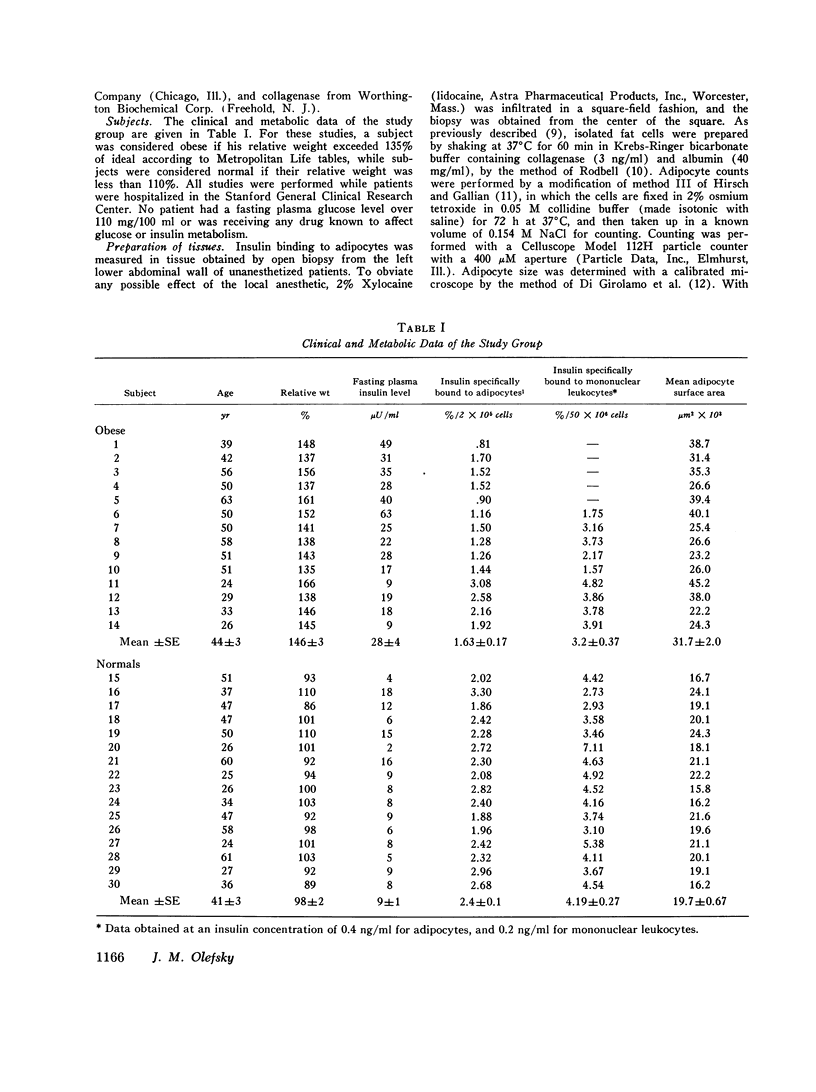
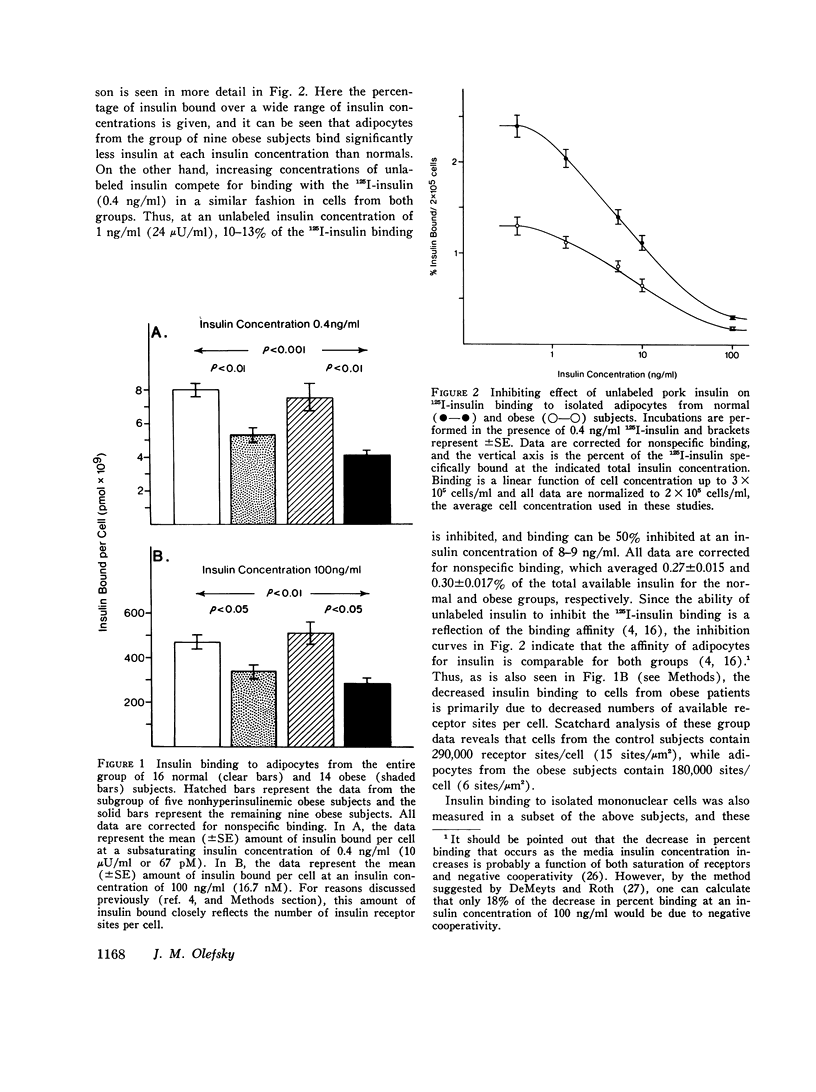
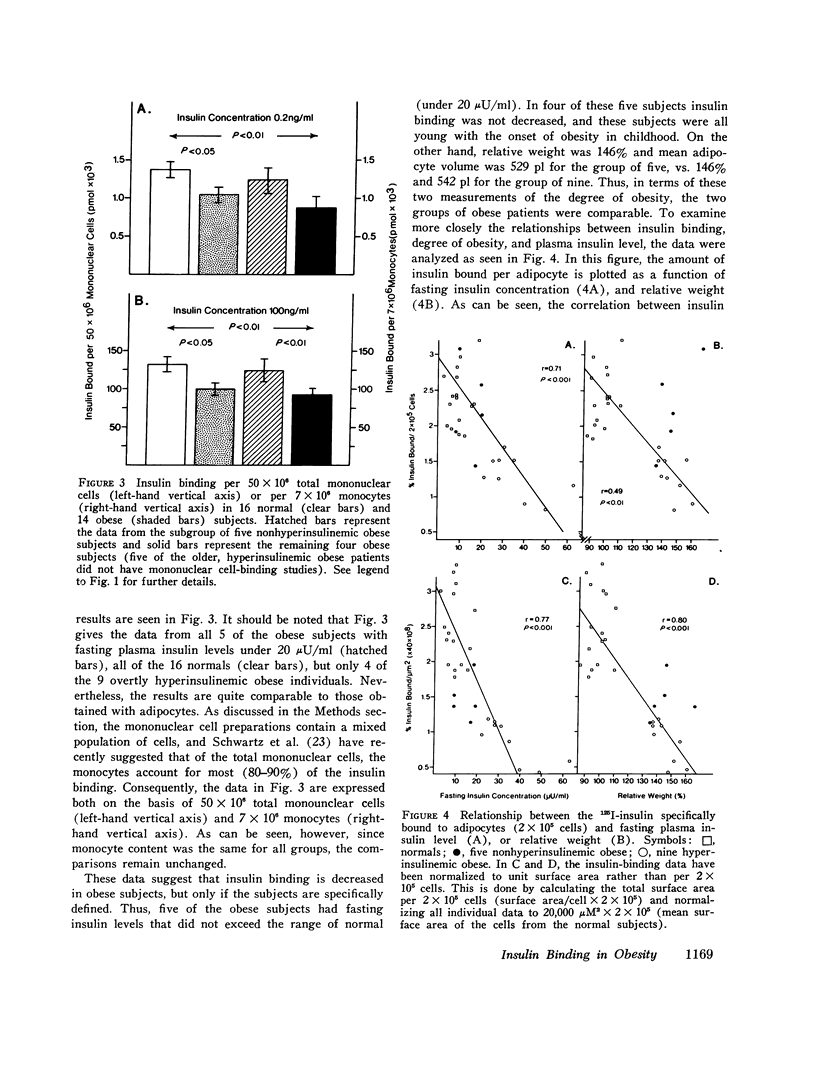
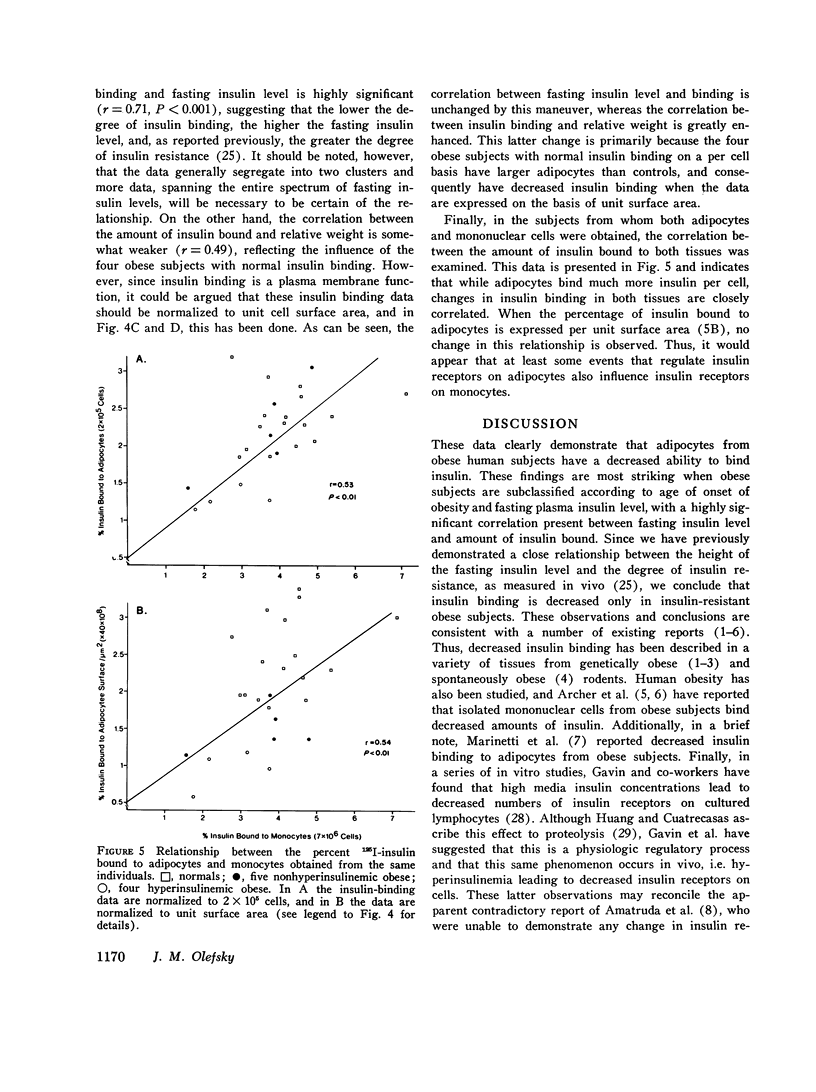
Insulin binding to isolated adipocytes from 16 normal and 14 obese patients was studied. The data indicated that, as a group, adipocytes from the obese patients bound significantly less insulin than normal. However, of the 14 obese patients, 5 were not hyperinsulinemic and 4 of these 5 subjects had normal insulin binding. These subjects were also younger, and had the onset of obesity in childhood. When these five patients were separated from the original 14 obese patients, enhanced differences in insulin binding to adipocytes were observed when normals and the remaining 9 obese subjects were compared. Similar findings were obtained with isolated circulating mononuclear cells from these same patients. Presumably the five normoinsulinemic obese patients were not insulin-resistant, and, thus, the data indicate that insulin binding to adipocytes was decreased only in insulin-resistant obese patients. This conclusion was strengthened by finding a highly significant correlation (r=-0.71, p less than 0.001) between insulin binding to adipocytes and fasting plasma insulin level, while a weaker correlation (r=-0.49,p less than 0.01) existed between insulin binding and degree of obesity. Finally, when insulin binding to adipocytes and mononuclear cells from the same individual was compared, a significant positive correlation was found (r=0.53,p less than 0.01). In conclusion: (a) insulin binding to adipocytes and mononuclear cells is decreased in cells from insulin-resistant obese patients; (b) a significant inverse relationship exists between fasting plasma insulin level and insulin binding to adipocytes; and (c) in obesity, events that affect insulin receptors on adipocytes similarly affect insulin receptors on mononuclear cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amatruda J. M., Livingston J. N., Lockwood D. H. Insulin receptor: role in the resistance of human obesity to insulin. Science. 1975 Apr 18;188(4185):264–266. doi: 10.1126/science.164059. [DOI] [PubMed] [Google Scholar]
- Archer J. A., Gorden P., Gavin J. R., 3rd, Lesniak M. A., Roth J. Insulin receptors in human circulating lymphocytes: application to the study of insulin resistance in man. J Clin Endocrinol Metab. 1973 Apr;36(4):627–633. doi: 10.1210/jcem-36-4-627. [DOI] [PubMed] [Google Scholar]
- Archer J. A., Gorden P., Roth J. Defect in insulin binding to receptors in obese man. Amelioration with calorie restriction. J Clin Invest. 1975 Jan;55(1):166–174. doi: 10.1172/JCI107907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Freychet P., Laudat M. H., Laudat P., Rosselin G., Kahn C. R., Gorden P., Roth J. Impairment of insulin binding to the fat cell plasma membrane in the obese hyperglycemic mouse. FEBS Lett. 1972 Sep 15;25(2):339–342. doi: 10.1016/0014-5793(72)80519-2. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Jen P., Freychet P. Insulin receptors in human circulating cells and fibroblasts. Proc Natl Acad Sci U S A. 1972 Mar;69(3):747–751. doi: 10.1073/pnas.69.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Huang D., Cuatrecasas P. Insulin-induced reduction of membrane receptor concentrations in isolated fat cells and lymphocytes. Independence from receptor occupation and possible relation to proteolytic activity of insulin. J Biol Chem. 1975 Oct 25;250(20):8251–8259. [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Olefsky J. M., Jen P., Reaven G. M., Alto P. Insulin binding to isolated human adipocytes. Diabetes. 1974 Jul;23(7):565–571. doi: 10.2337/diab.23.7.565. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Decreased insulin binding to lymphocytes from diabetic subjects. J Clin Invest. 1974 Dec;54(6):1323–1328. doi: 10.1172/JCI107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J., Farquhar J. W., Reaven G. Relationship between fasting plasma insulin level and resistance to insulin-mediated glucose uptake in normal and diabetic subjects. Diabetes. 1973 Jul;22(7):507–513. doi: 10.2337/diab.22.7.507. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Goldfine I. D., Roth J., Kahn C. R. Thymic lymphocytes in obese (ob-ob) mice. A mirror of the insulin receptor defect in liver and fat. J Biol Chem. 1974 Jul 10;249(13):4127–4131. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


