Abstract
2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2279) was developed previously as a selective high-affinity, non-nucleotide P2Y1 receptor (P2Y1-R) antagonist (J Med Chem 43:829–842, 2002; Br J Pharmacol 135:2004–2010, 2002). We have taken advantage of the N6-methyl substitution in the adenine base to incorporate [3H]methylamine into the synthesis of [3H]MRS2279 to high (89 Ci/mmol) specific radioactivity and have used this molecule as a radioligand for the P2Y1-R. [3H]MRS2279 bound to membranes from Sf9 insect cells expressing recombinant human P2Y1-R but not to membranes from wild-type Sf9 cells or Sf9 cells expressing high levels of recombinant P2Y2 or P2Y12 receptors. Equilibrium binding of [3H]MRS2279 to P2Y1-R expressed in Sf9 membranes was with a high affinity (Kd = 8 nM) essentially identical to the apparent affinity of MRS2279 determined previously in studies of P2Y1-R–promoted inositol phosphate accumulation or platelet aggregation. A kinetically derived Kd calculated from independent determinations of the rate constants of association (7.15 × 107 M−1 min−1) and dissociation (0.72 min−1) of [3H]MRS2279 also was in good agreement with the Kd derived from equilibrium binding studies. Competition binding assays with [3H]MRS2279 and P2Y1-R expressing Sf9 cell membranes revealed Ki values for the P2Y1-R antagonists MRS2279 (Ki = 13 nM), N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179; Ki = 84 nM), adenosine-3′, 5′-bisphosphate (Ki = 900 nM), and pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (Ki = 6 µM) that were in good agreement with antagonist activities of these molecules previously determined at the P2Y1-R in intact tissues. Moreover, [3H]MRS2279 also bound with high affinity (Kd = 4–8 nM) to Chinese hamster ovary (CHO) or 1321N1 human astrocytoma cells stably expressing the human P2Y1-R, but specific binding was not observed in wild-type CHO or 1321N1 cells. [3H]MRS2279 bound with high affinity (Kd = 16 nM) to a binding site on out-dated human platelets (5–35 receptors/platelet) and rat brain membranes (210 fmol/mg protein) that fit the expected drug selectivity of a P2Y1-R. Taken together, these results indicate that [3H]MRS2279 is the first broadly applicable antagonist radioligand for a P2Y receptor.
Physiological responses to extracellular nucleotides occur in most tissues (Dubyak and El-Moatassim, 1993; Ralevic and Burnstock, 1998). These effects occur through both ligand-gated ion channel P2X receptors and the G protein-coupled P2Y receptors. P2X receptors are activated predominantly by ATP (Khakh et al., 2000). In contrast, the individual P2Y receptors exhibit notable differences in agonist selectivity (Harden et al., 1998a,b), and the cognate agonists for these metabotropic receptors include not only ATP (P2Y11 receptor), but also ADP (P2Y1, P2Y12, and P2Y13 receptors), UTP (P2Y4 receptor), UDP (P2Y6 receptor), or both ATP and UTP (P2Y2 receptor). A diverse family of ectoenzymes and extracellular enzymes hydrolyze and interconvert extracellular nucleotides (Zimmermann, 1996), in some cases inactivating cognate agonists for one or more P2Y receptors while producing the cognate agonist of another P2Y receptor (Harden et al., 1997). Only a nascent knowledge has accrued of the functional relationship between the ectoenzymes capable of hydrolyzing extracellular nucleotides and the receptors capable of transducing their binding into cellular signaling responses.
The pharmacological armamentarium for P2Y receptors is limited. Few if any agonists exist that exhibit high-affinity selectivity among the P2Y receptors as well as resistance to ectoenzyme-catalyzed metabolism. Receptor-selective high-affinity antagonists of the various P2Y receptor subtypes also have not been generally available (Harden et al., 1998a; Khakh et al., 2000). Thus, pharmacological delineation of the role of a given receptor subtype in a given physiological response has often been difficult. Given the lack of effective pharmacological agents for the study of this important and broad class of signaling proteins, it is not surprising that generally useful and reliable radioligands that allow direct quantitation of P2Y receptors in mammalian tissues also have not been available.
We have made significant progress in the past 6 years in the chemical syntheses of receptor-selective antagonists of the P2Y1-R. This drug development originated from the observation that adenosine bisphosphate molecules were competitive antagonists of the P2Y1-R (Boyer et al., 1996a) and has evolved to the identification of at least three different series of non-nucleotide bisphosphate antagonists of these receptors (Camaioni et al., 1998; Kim et al., 2000, 2001; Nandanan et al., 2000). We recently reported that one of these molecules, MRS2279, exhibits a KB of approximately 10 nM for the turkey and human P2Y1-R (Boyer et al., 2002). Given the high affinity of this molecule and its high selectivity of association with the P2Y1-R versus other P2Y receptor family members, we hypothesized that a radioactive form of MRS2279 might provide a radioligand for direct quantitation and characterization of P2Y receptors in various tissues. Thus, [3H]MRS2279 was synthesized to high specific radioactivity. We report here that this molecule is a reliable radio probe for the P2Y1-R under conditions in which the receptor is either heterologously expressed in various cell types, solubilized and maintained in detergent solution in a soluble form, or natively expressed in human or rat tissues.
Materials and Methods
Precursor for Synthesis of MRS2279
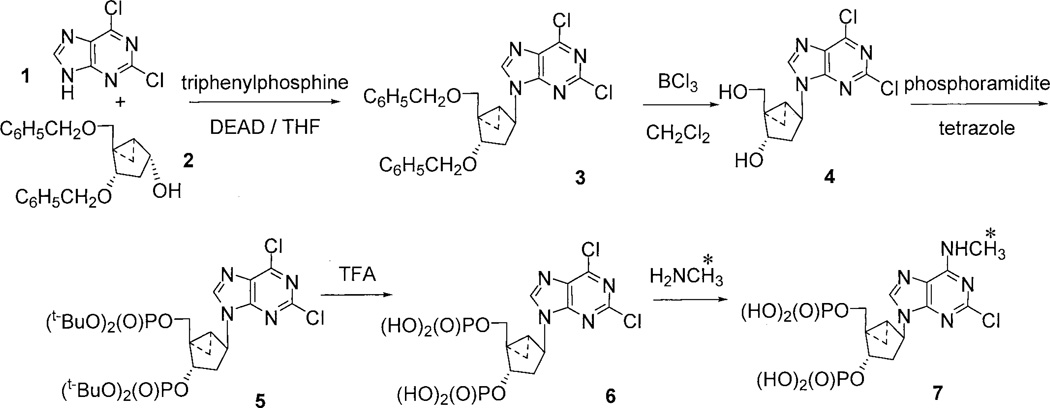
We described previously in detail the synthesis of MRS2279 (Nandanan et al., 2000). The presence of a methyl group in the N6 position of the adenine base of MRS2279 made possible the synthesis of radiolabeled MRS2279 using high specific activity [3H]methylamine. Therefore, we synthesized the bisphosphate molecule 6 as a precursor for synthesis of [3H]MRS2279 in a final step using [3H]methylamine (Fig. 1). 2,6-Dichloropurine 1 was coupled with dibenzylether 2 through a standard Mitsunobu procedure (PPh3, DEAD, THF, 0°C to room temperature, 2 h) to give 3 in 69% yield. The two O-benzyl protecting groups were removed by treatment of 3 with boron trichloride at low temperature, which afforded diol 4 in 60% yield. Bis-phosphorylation on diol 4 was carried out using the phosphoramidite method (Perich and Johns, 1988). Synthesis of the tert-butyl phosphate ester 5 was accomplished in 55% yield by treatment of diol 4 with di-tert-butyl diethylphosphoramidite and tetrazole in THF, followed by oxidation with m-chloroperbenzoic acid. Bis-phosphate 6, the precursor for [3H]MRS2279, was synthesized upon deprotection of the t-butyl groups under acidic conditions (5% trifluoroacetic acid in methylene chloride in 56% yield). The bis-phosphate was stored as the triethylammonium salt instead of ammonium salt due to the susceptibility of the 6-chloro group on the adenine moiety to attack by nucleophiles. Conditions were optimized for efficient conversion of 2,6-dichlorobis-phosphate 6 to MRS2279 by treatment with 40% methylamine in water. All materials were characterized by 1H NMR, 31P NMR, and by low- or high-resolution mass spectroscopy. The resulting spectral data were consistent with the structural assignments.
Fig. 1.
Synthesis of [3H]MRS2279. [3H]MRS2279 (molecule 7) was synthesized from the two precursor molecules (1 and 2) in a five step scheme described under Materials and Methods. *, the position of tritium labeling.
Radiochemical Synthesis of [3H]MRS2279
Molecule 6 was prepared as above, and preliminary syntheses were carried out to optimize synthesis of nonradioactive MRS2279 from the bisphosphate molecule 6 and methylamine. These conditions then were applied to the synthesis of [3H]MRS2279. [3H]Methylamine hydrochloride (145 mCi; specific activity, 88.98 Ci/mmol, 1.63 µmol) was dissolved in water (0.1 ml), and the pH was adjusted to 9 with diisopropylethylamine (3 µl). Compound 6 (0.53 mg, 1.12 µmol) was added, and the resulting mixture, pH 9.0, was stirred for 72 h at room temperature. The reaction mixture was subjected to preparative reverse phase HPLC using an Ace C18, 5 µm, 100 Å, 10 × 250 mm column and a linear gradient of 1% triethylamine acetate, pH 4, with acetonitrile (7 to 30% over 30 min). Approximately 3.0 mCi of [3H]MRS2279 was purified with a retention time of approximately 20 min.
P2Y1-R Expression in Sf9 Insect Cells
Recombinant baculoviruses encoding the human P2Y1-R, P2Y2-R, or P2Y12-R flanked by a carboxyl-terminal hexahistidine tag and an amino-terminal signal sequence (MKTIIALSYIFCLVFA) followed by a FLAG epitope (DYKDDDA) were constructed by established protocols using the pVL-1392 transfer vector. Cultures of Sf9 cells (1.2 liter; −1.5 × 106 cells/ml) were infected with recombinant baculovirus (multiplicity of infection = 2) and incubated in spinner flasks for 48 h at 27°C. Sf9 cell plasma membranes expressing the tagged receptors were prepared by the following protocol. Cultures were centrifuged for 10 min at 550g (JA-14 rotor; Beckman Coulter, Fullerton, CA), and the cell pellet was resuspended in 120 ml of cavitation buffer (20 mM Tris, pH 8.0, 100 mM NaCl, 2 mM β-mercaptoethanol, 100 µg/ml phenyl-methylsulfonyl fluoride, 100 µM benzamidine, and 100 µg/ml N-tosyl-l-phenylalanine chloromethyl ketone), incubated at 600 psi for 1 h at 4°C in a Parr bomb, and disrupted by rapid release of N2 pressure. The lysed cells were centrifuged at 450g (JS-4.2 rotor; Beckman Coulter) for 10 min, the pellet was discarded, and the supernatant was centrifuged at 30,000g (Beckman JA-14 rotor) for 30 min. The high-speed pellet was resuspended in 400 ml of cavitation buffer and centrifuged again at 30,000g for 30 min. The final pellet was resuspended to approximately 4 mg of membrane protein/ ml in freezing buffer (cavitation buffer with 250 mM sucrose substituted for NaCl). The membranes were frozen in aliquots at −80°C.
Platelet Preparation
Outdated human platelets were obtained from the University of North Carolina blood bank 6 days after donation. Platelets were washed once by centrifugation (10 min at 1000g; JS-4.2 rotor) in a buffer consisting of 10 mM HEPES, pH 7.4, 137 mM NaCl, 2.7 mM MgCl2, 3 mM NaH2PO4, 5 mM glucose, 0.2% bovine serum albumin, and 0.05 U/ml apyrase. The washed platelets were resuspended to a final concentration of approximately 3 × 1010 platelets/ml and assayed intact or the platelets were ruptured either by freeze/thaw, sonication, or nitrogen cavitation, and washed platelet membranes were prepared by repeated centrifugation and resuspension in the above buffer without apyrase.
Rat Brain Membranes
Whole brains were obtained from adult female Sprague-Dawley rats and homogenized (1 g wet weight/20–30 volumes) in 20 mM HEPES, pH 7.5, 150 mM NaCl, and 2 mM MgCl2 using a Polytron tissue disrupter (Brinkmann Instruments, Westbury, NY). The homogenate was centrifuged at 35,000g for 10 min, and the resultant pellet was resuspended in the same buffer. This centrifugation step was repeated two more times. The final resuspension was in 5 volumes of buffer and stored frozen as multiple aliquots at −80°C until needed.
Stable Expression of the P2Y1-R in Mammalian Cell Lines
1321N1 human astrocytoma (Schachter et al., 1996) and CHO-K1 (Alvarado-Castillo et al., 2002) cell lines stably expressing hP2Y1-R were established as described previously by infection of the respective cell lines with recombinant retrovirus encoding the human P2Y1-R.
Radioligand Binding Assay
Membranes typically were incubated at 4°C with 5 to 7.5 nM [3H]MRS2279 (approximately 20,000–30,000 cpm) in 20 mM Tris, pH 7.5 at 4°C, 145 mM NaCl, and 5 mM MgCl2 in a volume of 50 µl. Saturation binding isotherms used concentrations of [3H]MRS2279 ranging from 0.5 to 150 nM and were carried out in a volume of 25 µl. Specific binding was usually defined as total [3H]MRS2279 binding minus binding occurring in the presence of a 10 µM concentration of the P2Y receptor-specific (Boyer et al., 1998) antagonist MRS2179, or in some experiments, 10 µM 2MeSADP. Incubations were for 15 to 60 min in an ice-water bath. Binding reactions were terminated by addition of 4 ml of ice-cold wash buffer (10 mM Tris, pH 7.5 at 4°C, and 145 mM NaCl) and rapid filtration over GF/A glass fiber filters. Each filter was washed with an additional 4 ml of ice-cold wash buffer. Radioactivity was quantitated by liquid scintillation spectrometry.
Digitonin Extraction of P2Y1-Sf9 Membranes and Partial Purification of the Recombinant P2Y1-R
Frozen P2Y1-Sf9 membranes were thawed and centrifuged at 46,000g for 30 min (JA20 rotor; Beckman Coulter). The membrane pellet was resuspended to a final concentration of 2 to 4 mg of membrane protein/ml in extraction buffer (20 mM HEPES, pH 8.0, 10% glycerol, 70 mM NaH2PO4, 100 µM ADP, 5 mM β-mercaptoethanol, 1% digitonin, 15 mM imidazole, 500 mM NaCl, 100 µM benzamidine, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 100 µg/ml phenylmethylsulfonyl fluoride, and 50 µg/ml N-tosyl-l-phenylalanine chloromethyl ketone) and incubated for 2 h at 4°C with constant stirring. The extracted membranes were centrifuged for 1 h at 100,000g (Type 35 rotor; Beckman Coulter). The supernatant was batch-loaded on Ni-NTA agarose (1 ml bed volume) for 2 h at 4°C and the P2Y1-R was eluted from the resin with 250 mM imidazole.
Soluble Radioligand Binding Assay
Digitonin-solubilized P2Y1-R was incubated for 60 min at 4°C with 1 to 80 nM [3H]MRS2279 in 20 mM Tris, pH 7.5 at 4°C, 145 mM NaCl, and 1% digitonin in a 25-µl volume. Bound radioligand was separated from free [3H]MRS2279 by Sephadex G-50 spin column [3-ml bed volume in Poly-Prep columns (Bio-Rad, Hercules, CA)] chromatography. G-50 columns were equilibrated with assay buffer and centrifuged at 900g (JS-4.2 rotor) for 10 s immediately before adding 20 µl of the binding reaction to the center of a column. The bound radioligand was then eluted by centrifugation for 5 min at 1400g (JS-4.2 rotor). The eluate was collected directly in polypropylene minivials and quantified by liquid scintillation spectrometry.
ATP and ADP Metabolism by Sf9 Membranes
P2Y1-Sf9 membranes at the concentration (10 µg/50 µl) used in [3H]MRS2279 binding assays were incubated with 10 µM ATP or 10 µM ADP for various times at 4°C. The incubation was stopped by transfer of aliquots into 5% trichloroacetic acid. Nucleotide concentration in the extract was determined after etheno-derivation as described previously (Huang et al., 2001).
Data Analysis
All experiments were carried out in triplicate or quadruplicate assays and were repeated at least three times. Data are presented as mean ± S.E.M. from combined multiple experiments or in some cases as a data set from a typical experiment.
Results
An iterative set of chemical syntheses, structure activity testing, and model building (Boyer et al., 1998, 2002; Camaioni et al., 1998; Kim et al., 2000; Nandanan et al., 1999, 2000) led to identification of a high affinity antagonist of the P2Y1-R. This ring-constrained (N)-methanocarba bisphosphate molecule, MRS2279 (molecule 7, Fig. 1), does not interact with other P2Y receptors (Boyer et al., 2002), and we anticipate that this non-nucleotide molecule also exhibits little if any binding to the multifarious nucleotide binding proteins in the genome of mammalian species. Thus, we reasoned that MRS2279 potentially would satisfy the characteristics needed for a generally useful radioligand for quantitation and characterization of the P2Y1-R. This hypothesis was tested as described below.
The presence of an N6-methyl group in MRS2279 allowed us to use high-specific activity [3H]methylamine to synthesize [3H]MRS2279 from the precursor molecule 6 as illustrated in Fig. 1 and as described under Materials and Methods. Pure radioligand with a specific radioactivity of 88.98 Ci/mmol was obtained. Additional details for synthesis of nonradioactive MRS2279 and the precursor molecules are provided in our previous studies (Kim et al., 2000, Nandanan et al., 2000).
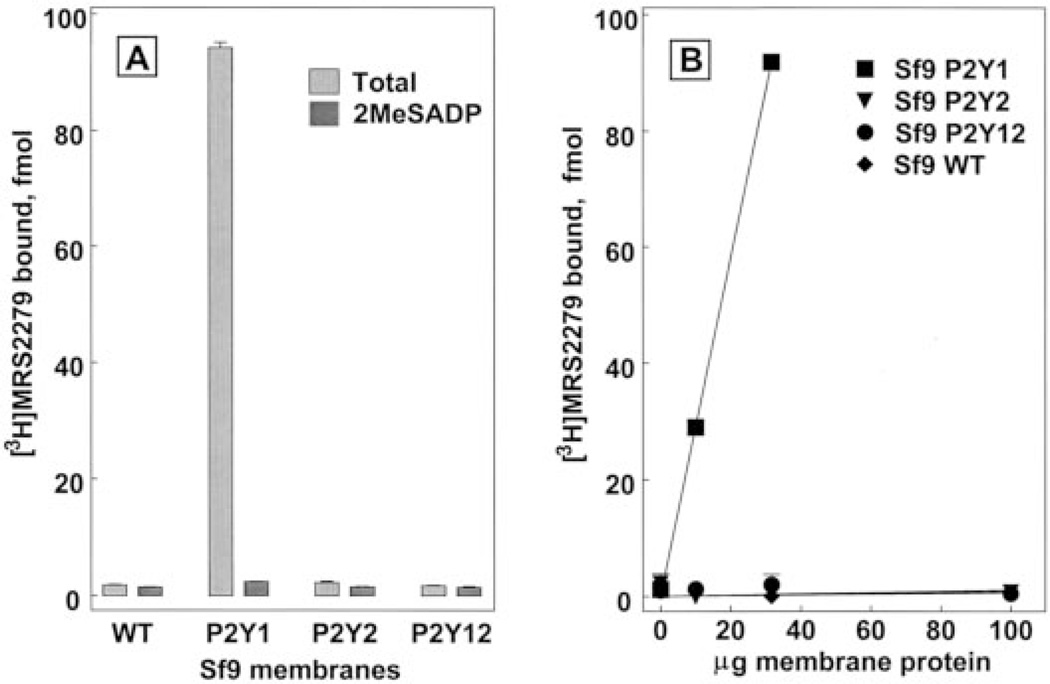
The potential use of [3H]MRS2279 as a P2Y1-R radioligand was tested initially using membranes prepared from wild-type Sf9 insect cells or membranes prepared from Sf9 cells infected with recombinant baculoviruses for the human P2Y1, P2Y2, or P2Y12 receptors. Specific binding of [3H]MRS2279 was defined in these experiments using the P2Y1-R agonist 2MeSADP at an anticipated saturating concentration of 10 µM. Essentially no binding of [3H]MRS2279 was detected either in the absence or presence of 10 µM 2MeSADP in membranes from wild-type Sf9 cells or in membranes from P2Y2 or P2Y12 receptor baculovirus-infected cells (Fig. 2A). High-level expression of the P2Y2 and P2Y12 receptors was confirmed in these membranes by immunoblots (data not shown). In contrast, specific binding (3.5 pmol/mg protein at 6 nM [3H]MRS2279) accounted for 98% of total ligand binding in P2Y1-R expressing membranes (Fig. 2A), and the amount of binding was directly proportional to the amount of membrane in the assay (Fig. 2B). Thus, [3H]MRS2279 binds to Sf9 membranes in a manner that is absolutely dependent on the expression of the P2Y1-R, but not other adenine nucleotide-recognizing P2Y receptors. As such, P2Y1-Sf9 membranes were used to delineate the properties of binding of [3H]MRS2279 to the human P2Y1-R.
Fig. 2.
P2Y1-R selective binding of [3H]MRS2279 in membranes prepared from Sf9 cells. Sf9 membranes prepared from wild-type cells or cells expressing the recombinant human P2Y1, P2Y2, or P2Y12 receptors (30 µg/assay, A) were incubated with 6 nM [3H]MRS2279 in the absence ( ) or the presence (
) or the presence ( ) of 10 µM 2MeSADP. The specific binding of [3H]MRS2279 (defined with 10 µM 2MeSADP) was determined over the indicated range of membrane protein for each preparation (B).
) of 10 µM 2MeSADP. The specific binding of [3H]MRS2279 (defined with 10 µM 2MeSADP) was determined over the indicated range of membrane protein for each preparation (B).
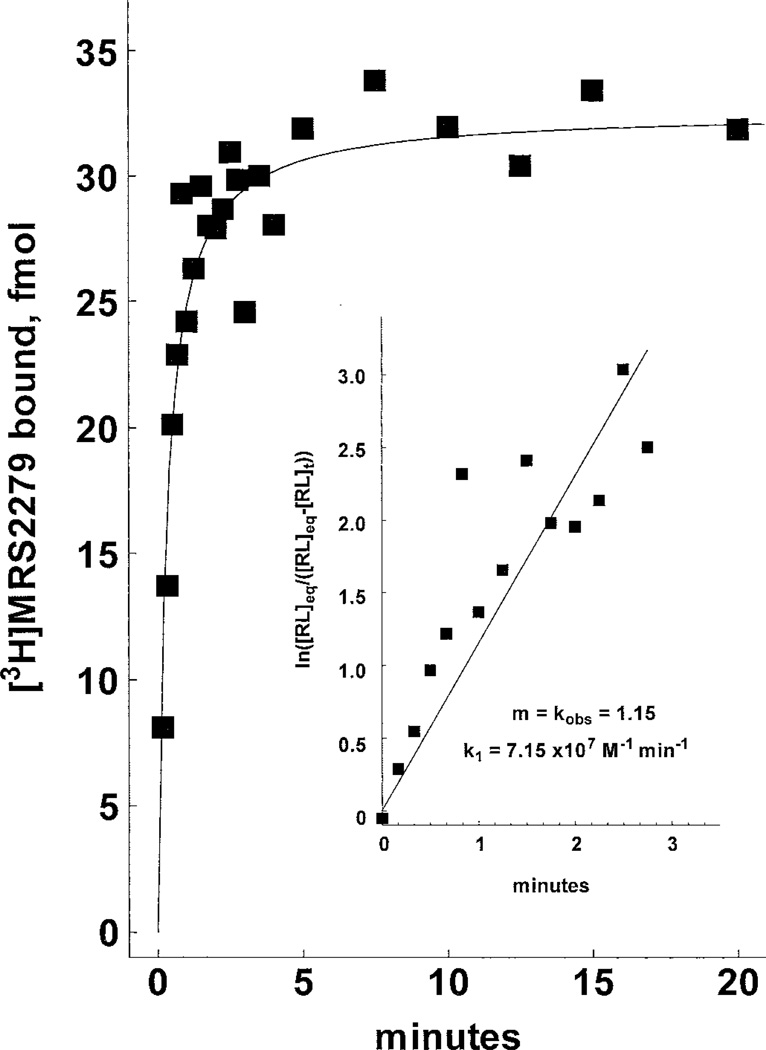
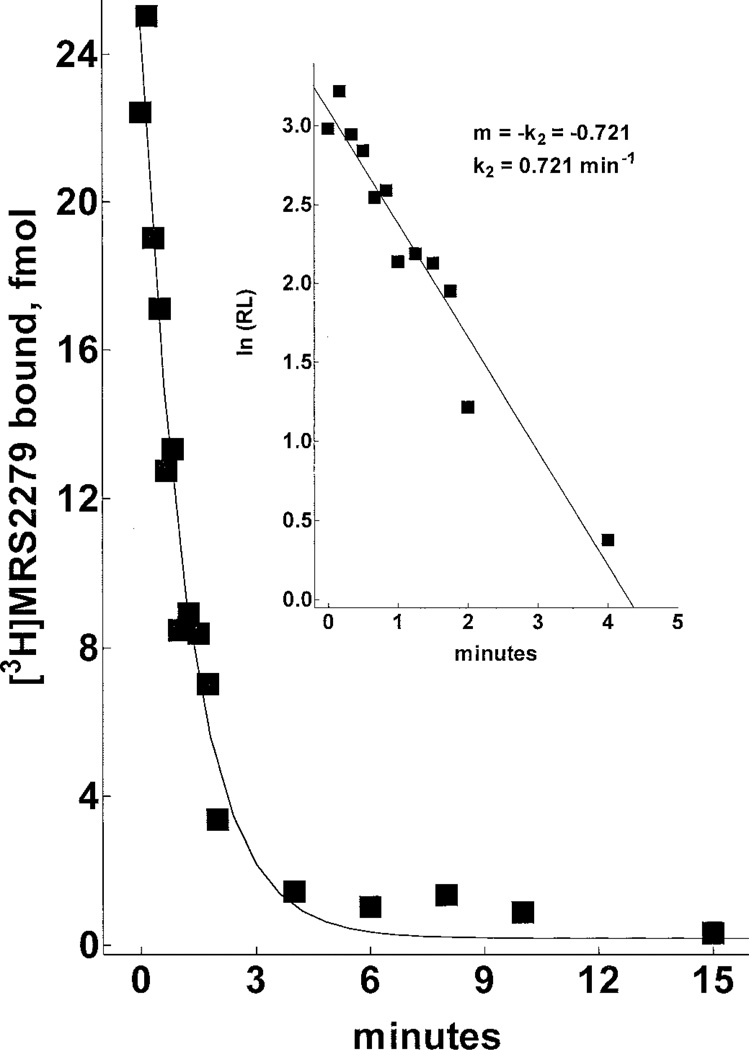
The receptor binding kinetics of [3H]MRS2279 were defined in preliminary time course experiments performed at 4°C versus 30°C. Although maximal binding was similar at both temperatures, the binding of [3H]MRS2279 at steady state was somewhat more stable with time at 4°C than at 30°C. Therefore, subsequent assays were primarily performed at 4°C. As illustrated in Fig. 3, binding of 5 nM [3H]MRS2279 to P2Y1-Sf9 membranes was rapid, reaching an apparent steady state within 5 min that was maintained for at least 60 min. Rate plots (Fig. 3, inset) demonstrated a linear relationship between radioligand binding and time. A rate constant for association (k1) of 7.15 × 107 M−1min−1 was calculated from these data. The time course of dissociation of [3H]MRS2279 from the receptor also was determined. Addition of 10 µM 2MeSADP subsequent to attainment of steady state binding resulted in rapid dissociation (t1/2 ≈ 1.5 min) of [3H]MRS2279. Binding was completely reversible within 10 to 15 min to the level of nonspecific binding (Fig. 4). First order-rate plots revealed a linear relationship between binding and time (Fig. 4, inset), and a first-order rate constant (k2) for dissociation of 0.72 min−1 was calculated. A kinetically derived Kd (k2/k1 = Kd) of 10 nM resulted from these independent determinations of the forward and reverse rate constants.
Fig. 3.
Association rate kinetics of [3H]MRS2279 binding in P2Y1-Sf9 membranes. P2Y1-Sf9 membranes (10 µg/assay) were incubated for the indicated times at 4°C with 5.8 nM [3H]MRS2279 and with or without 10 µM 2MeSADP before rapid filtration through GF/A filters as described under Materials and Methods. The data shown are specific binding of the radioligand. Inset, pseudo–first-order rate plot.
Fig. 4.
Dissociation rate kinetics of bound [3H]MRS2279 in P2Y1-Sf9 membranes. P2Y1-Sf9 membranes (7 µg/assay) were incubated to steady state (25 min at 4°C) with 6.3 nM [3H]MRS2279. At time 0, a saturating concentration of competing drug (10 µM 2MeSADP) was added and the reactions were terminated at the indicated times by rapid filtration through GF/A filters as described under Materials and Methods. Inset, first-order rate plot.
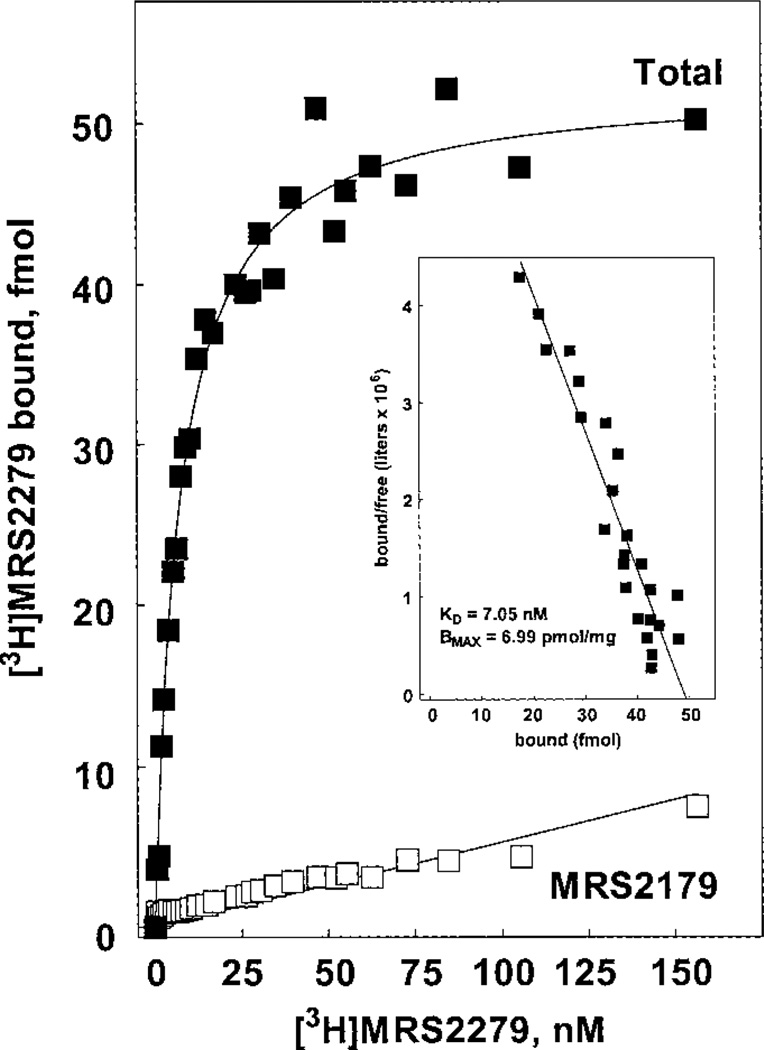
Saturation binding kinetics were determined by varying the concentration of [3H]MRS2279 over a 300-fold range. As illustrated in the saturation and Scatchard plots in Fig. 5, [3H]MRS2279 binding was saturable and occurred to an apparently homogeneous population of sites with a Kd of 7.8 ± 1.1 nM (n = 3 independent determinations) and a Bmax of 6.8 ± 0.2 pmol/mg of protein in the P2Y1-Sf9 membranes. A KB of 8 nM was obtained in our earlier studies of the activity of MRS2279 as a competitive antagonist at the human P2Y1-R stably expressed in 1321N1 human astrocytoma cells (Boyer et al., 2002). Thus, the Kd values obtained in equilibrium binding determinations with [3H]MRS2279 were in excellent agreement with the kinetically obtained Kd for [3H]MRS2279 and the functionally obtained KB of nonradioactive MRS2279. The saturation binding experiments also reveal that the recombinant P2Y1-R is expressed to very high levels in Sf9 cells.
Fig. 5.
Saturation binding isotherm for [3H]MRS2279 binding in P2Y1-Sf9 membranes. P2Y1-Sf9 membranes (7 µg/assay) were incubated for 60 min at 4°C with the indicated concentrations (0.6 – 156 nM) of [3H]MRS2279 without (■) or with (□) the P2Y1-R antagonist MRS2179 (30–300 µM). Inset, Scatchard transformation of the data.
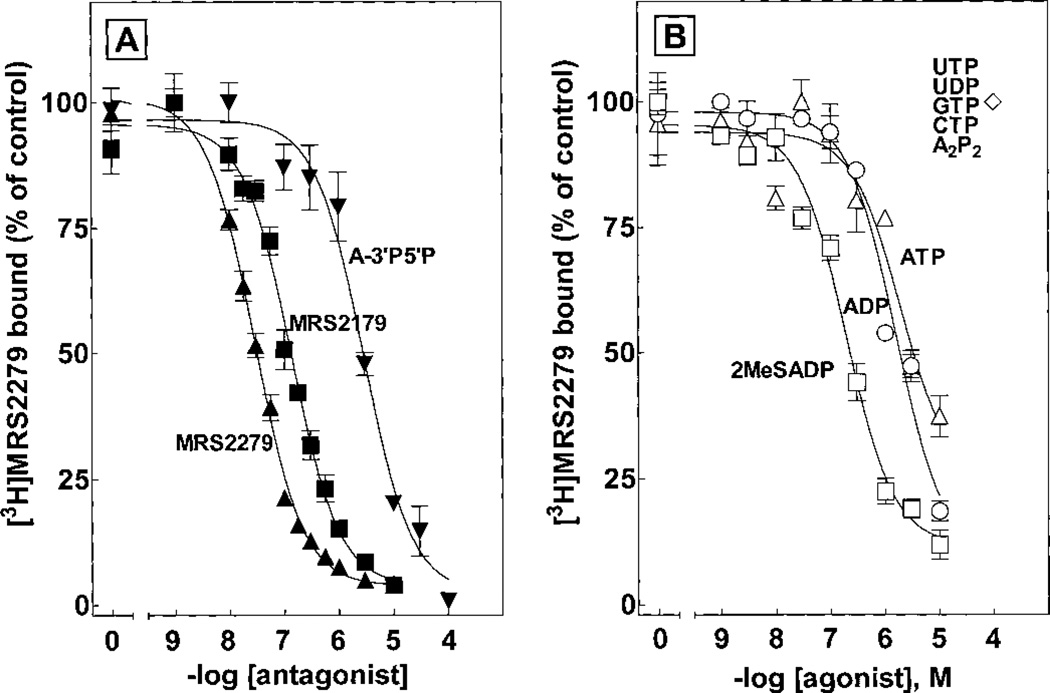
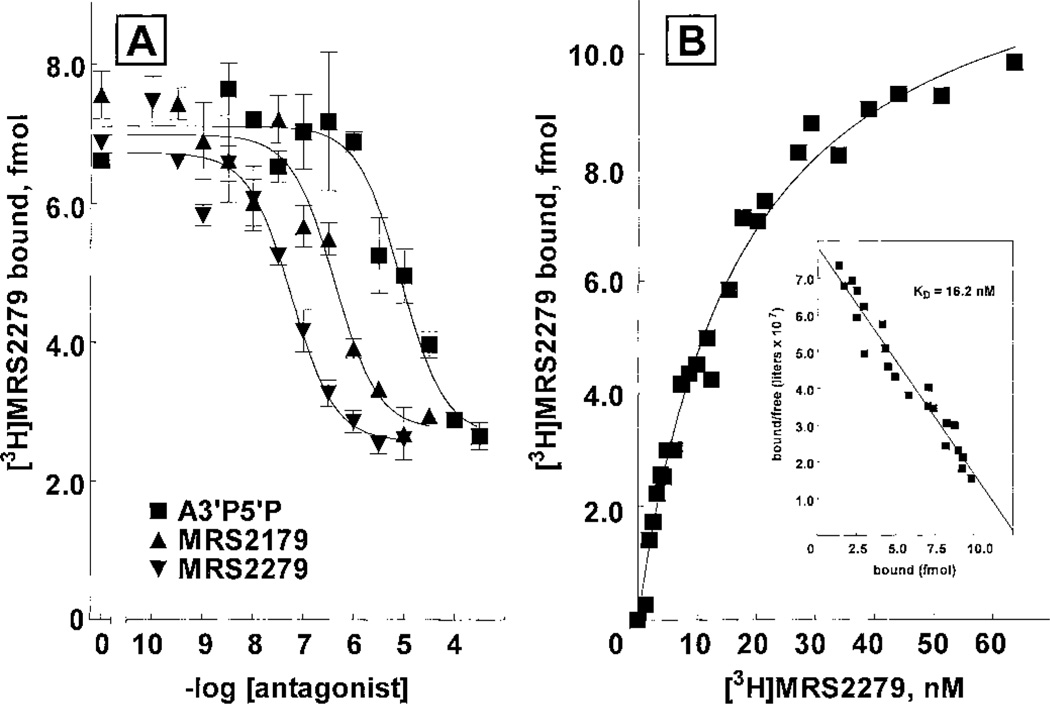
The pharmacological selectivity of the binding of [3H]MRS2279 to P2Y1-Sf9 membranes was determined in a series of competition curves with molecules known to bind to the P2Y1-R and other P2Y receptors (Fig. 6A and Table 1). Antagonist molecules previously shown to interact with the P2Y1-R exhibited Ki values similar to the KB values of these molecules determined in experiments measuring their capacity to inhibit P2Y1-R signaling. Thus, a Ki value of 13 nM was obtained for MRS2279 and the Ki value of a similar high-affinity bisphosphate molecule MRS2179 developed by our group (Boyer et al., 1998) was 84 nM (Table 1). In contrast, one of the originally identified adenosine bisphosphate antagonists (Boyer et al., 1996a), adenosine 3′,5′-bisphosphate, exhibited a Ki of 1.5 µM. Similarly, PPADS and suramin, which are antagonists at the P2Y1-R as well as many other P2Y and P2X receptors, exhibited Ki values of 6 and 5 µM, respectively.
Fig. 6.
Pharmacological selectivity of P2Y1-R antagonists and agonists for inhibition of [3H]MRS2279 binding in P2Y1-Sf9 membranes. P2Y1-Sf9 membranes were incubated 20 min at 4°C with 5 nM [3H]MRS2279 and the indicated concentrations of the antagonists MRS2279 (▲), MRS2179 (■), and A3′P5′P (▼) (A) or the agonists 2MeSADP (□), ADP (○), and ATP (△) (B). ◇, nucleotides that did not inhibit [3H]MRS2279 binding.
TABLE 1.
Pharmacological selectivity of [3H]MRS2279 binding in P2Y1-Sf9 membranes
| Antagonist | Ki (n = 3–5) | KB | Reference |
| nM | nM | ||
| MRS2279 | 13 ± 3 | 8 | Boyer et al. (2002) |
| MRS2179 | 84 ± 27 | 102 | Boyer et al. (1998) |
| A3′P5′P | 1,500 ± 400 | 900 | Boyer et al. (1996a) |
| PPADS | 6,200 ± 2,600 | 1200 | Boyer et al. (1994) |
| Suramin | 4,900 ± 1,600 | ||
| Agonist | IC50 (n = 3–6) | ||
| nM | |||
| 2MeSADP | 106 ± 51 | ||
| 2MeSATP | 112 ± 25 | ||
| ADP | 643 ± 79 | ||
| ATP | 777 ± 350 | ||
| ATPγS | 3,200 ± 1,700 | ||
ATPγS, adenosine-5′-O-(3-thio)triphosphate.
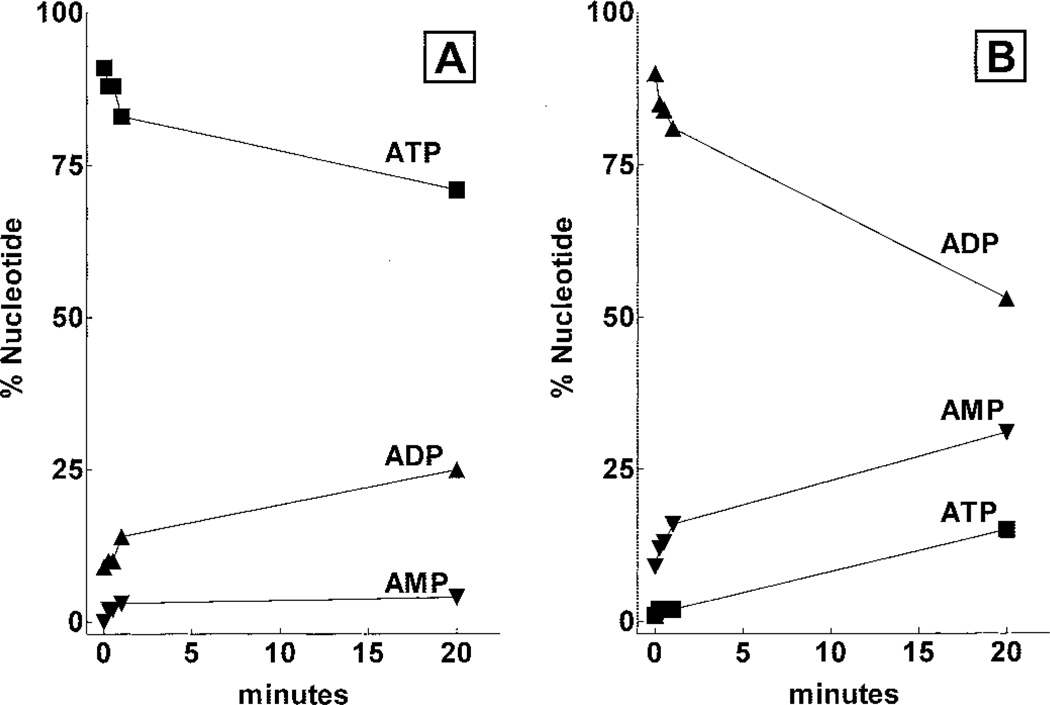
The binding of nucleotide agonists of the P2Y1-R also was studied with P2Y1-Sf9 membranes (Fig. 6B and Table 1). The order of potency for inhibition of [3H]MRS2279 binding was 2MeSADP > 2MeSATP > ADP > ATP > adenosine-5′-O-(3-thio)triphosphate. In contrast UTP, UDP, GTP, CTP, and A2P2 had no effect on [3H]MRS2279 binding at concentrations as high as 100 µM. We and others have previously reported that the human P2Y1-R exhibits high selectivity for adenosine diphosphate molecules and that relative effects of ATP or 2MeSATP on P2Y1-R signaling are difficult to establish because of the possible (and difficult to quantitate at the level of the receptor) conversion of ATP to ADP. Thus, we were concerned that the Ki values determined for ATP in competition binding experiments with [3H]MRS2279 were erroneous because of conversion of ATP to ADP during incubation of the nucleotides with membranes. To assess this possibility we carried out an experiment in which the rates of hydrolysis of ATP and ADP were determined with P2Y1-Sf9 membranes under the conditions of the radioligand binding assay (Fig. 7). ATP hydrolysis occurred under these conditions, and approximately 20% of the beginning nucleotide was in the form of ADP after a 20-min incubation. ADP also was metabolized with a 50% decrease in diphosphate and a coincidental appearance of AMP and ATP occurring during a 20-min incubation. These results are consistent with the presence of both ATPase and adenylate kinase activities in the membrane preparation. Moreover, these results indicate that calculation of reliable Ki values for nucleotide agonists is not feasible under the conditions of these assays although incubations were at 4°C with small amounts (7 µg/assay) of Sf9 cell membrane protein.
Fig. 7.
Metabolism of ATP and ADP by Sf9 membranes. Sf9 membranes (10 µg/assay) were incubated with 10 µM ATP (A) or 10 µM ADP (B) under conditions identical to those of the competition assays shown in Fig. 6. At the indicated times, the reactions were terminated by the addition of 5% TCA and the concentrations of ATP (■), ADP (▲), and AMP (▼) were determined by HPLC analysis as described under Materials and Methods.
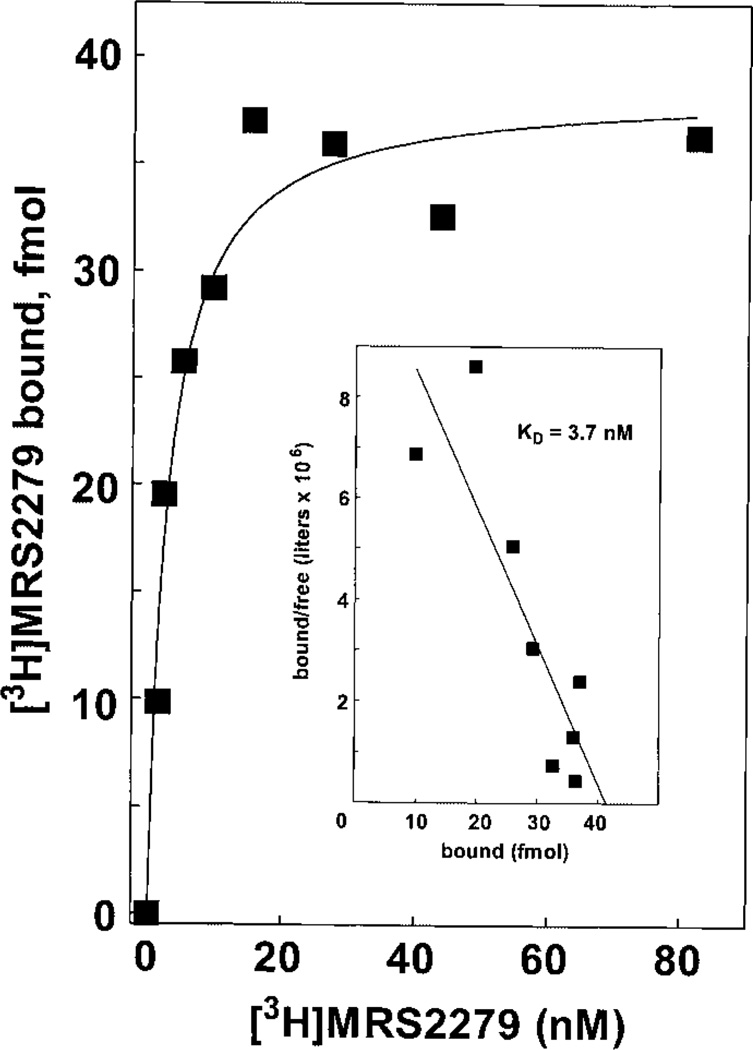
The results presented thus far indicate that [3H]MRS2279 is an excellent radioligand for the human P2Y1-R expressed at high levels in Sf9 cells. In other studies, we have solubilized the human P2Y1-R and purified it to near homogeneity (G. L. Waldo and T. K. Harden, in preparation). Thus, it also was important to establish the usefulness of [3H]MRS2279 as a radioligand for the P2Y1-R solubilized from Sf9 membranes using the detergent digitonin. A saturation binding isotherm illustrated high-affinity saturable binding of [3H]MRS2279 to soluble human P2Y1-R in the presence of digitonin (Fig. 8). The Kd of 4 nM for the soluble receptor compares favorably with that determined for the membrane receptor.
Fig. 8.
Saturation binding isotherm for [3H]MRS2279 binding to digitonin solubilized P2Y1-R. P2Y1-R were partially purified by nickel-nitrilotriacetic acid chromatography from a digitonin extract of P2Y1-Sf9 membranes as described under Materials and Methods. The soluble P2Y1-R was incubated for 60 min at 4°C with the indicated concentrations (1–80 nM) of [3H]MRS2279. Bound radioligand was separated from free radioligand by Sephadex G-50 chromatography. Data are plotted as specific binding. Inset, Scatchard transformation of the data.
We and others have used 1321N1 human astrocytoma cells as a null cell line for stable expression and study of P2Y1-R and other P2Y receptors. As such, we tested [3H]MRS2279 with membranes isolated from wild-type 1321N1 human astrocytoma cells and with membranes prepared from 1321N1 cells stably expressing the human P2Y1-R. No specific binding of [3H]MRS2279 was observed in membranes from wild-type 1321N1 cells, but specific binding occurred in the P2Y1-R expressing 1321N1 cells (Table 2). The Kd for [3H]MRS2279 (8 nM) was similar to that observed with the Sf9 insect cell-expressed P2Y1-R, and the Bmax of 0.4 pmol/mg of protein is in the range of recombinant protein expression expected after retrovirus-mediated expression in mammalian cells. We also have isolated clonal cell lines from CHO cells transfected with a human P2Y1-R expression vector. Although wild-type CHO cells do not exhibit significant [3H]MRS2279 binding, membranes prepared from the P2Y1-R–expressing cell line bound the radioligand with a Kd of 4 nM and Bmax of 1.1 pmol/mg protein.
TABLE 2.
KD and Bmax values for [3H]MRS2279 binding in CHO-K1 and 1321N1 human astrocytoma cells stably expressing the recombinant human P2Y1-R
| Cell type | KD | Bmax |
|---|---|---|
| nM | pmol/mg | |
| P2Y1 CHO | 4.3 | 1.1 |
| P2Y1 132 | 8.4 | 0.4 |
| WT CHO | N.D. | N.D. |
| WT 132 | N.D. | N.D. |
WT, wild-type; N.D., specific binding not detected.
The evolution of a P2Y1-R binding assay with [3H]MRS2279 in P2Y1-Sf9 membranes was effected with the ultimate goal of applying this assay to physiologically relevant tissues. Therefore, we examined the binding properties of [3H]MRS2279 to intact out-dated human platelets or platelet membranes. MRS2279, MRS2179, and adenosine 3′,5′ bisphosphate inhibited [3H]MRS2279 binding with an order of potency similar to that expected from previous studies of the human platelet P2Y1-R (Fig. 9A). 2MeSADP and ADP (IC50 = 1 and 7 µM, respectively) also potently inhibited [3H]MRS2279 binding to intact platelets and platelet membranes (data not shown). For the reasons of nucleotide metabolism discussed above, we did not attempt calculation of Ki values from these competition curves. Saturable binding of [3H]MRS2279 was observed (Fig. 9B) in both intact platelets and platelet membranes. A representative saturation-binding isotherm in intact platelets is presented in Fig. 9B. An average Kd of 23 ± 6 nM (n = 7 experiments) was obtained for intact platelets. A value of 5 to 35 receptors per platelet was determined with different out-dated platelet preparations obtained from multiple donors of various blood types. The Bmax for [3H]MRS2279 binding in platelet membranes was 170 fmol/mg protein.
Fig. 9.
[3H]MRS2279 binding in intact human platelets. Freshly outdated human platelets from a single donor with blood type B (A) or blood type O (B) were washed as described under Materials and Methods. Approximately 109 washed platelets/assay were incubated 30 min at 4°C with 6 nM [3H]MRS2279 and increasing concentrations of the P2Y1-R antagonists A3′P5′P (■), MRS2179 (▲), or MRS2279 (▼) (A). Approximately 109 washed platelets/assay were incubated for 50 min at 4°C with the indicated concentrations (1–65 nM) of [3H]MRS2279 (B). The data are plotted as specific binding. B, inset, Scatchard transformation of the data.
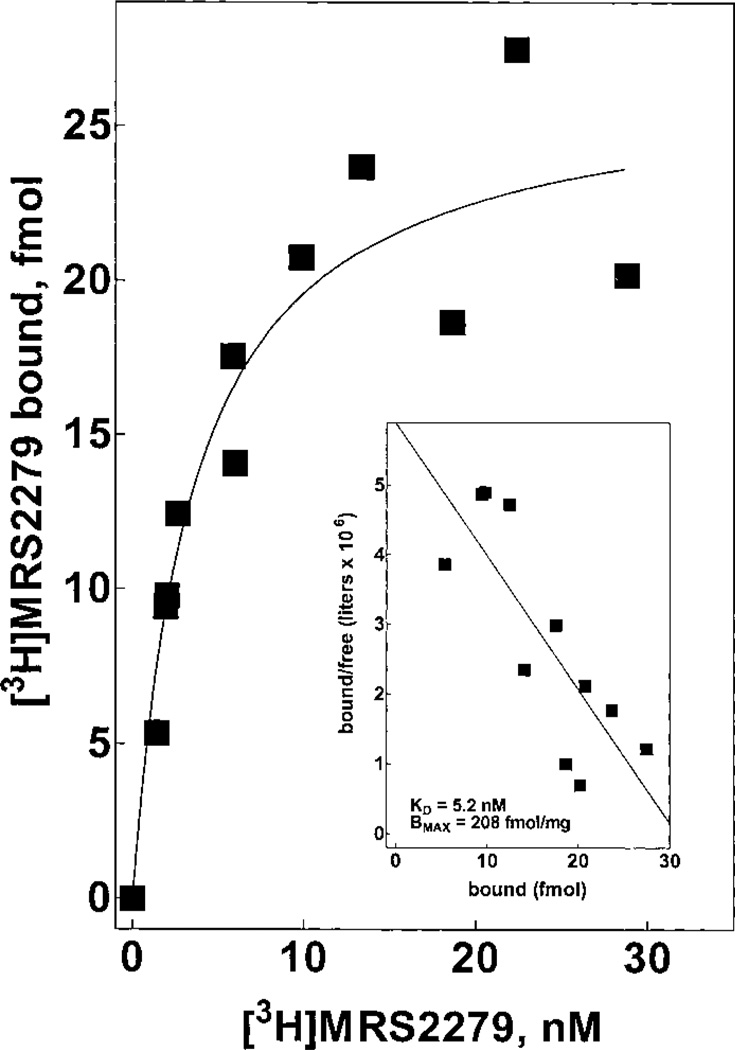
The properties of the P2Y1-R are similar across mammalian and avian species. Therefore, we anticipated that results obtained with [3H]MRS2279 and the human P2Y1-R would be applicable to other mammalian P2Y1-R. A key goal of these studies was to extend this binding assay to rat tissues. As such, we examined the binding properties of [3H]MRS2279 in rat brain membranes. [3H]MRS2279 bound with high affinity (Kd = 5 nM) to rat brain membranes (Fig. 10). The Bmax of 208 fmol/mg of protein compares well with the range of 50 to 500 fmol/mg protein observed for many other G protein-coupled receptors in rat brain. Given the very rapid hydrolysis of nucleotides by rat brain membranes, we did not analyze the effect of nucleotides on [3H]MRS2279 binding. MRS2279, MRS2179, and PPADS all inhibited [3H]MRS2279 binding to brain membranes with Ki values similar to those observed with the recombinant human P2Y1-R heterologously expressed in Sf9 insect cells (data not shown).
Fig. 10.
Saturation binding isotherm for [3H]MRS2279 binding in rat brain membranes. Rat brain membranes (~150 µg/assay) were incubated for 30 min at 4°C with the indicated concentrations (1–30 nM) of [3H]MRS2279. The data are plotted as specific binding. Inset, Scatchard transformation of the data.
Discussion
MRS2279 was developed by our laboratories as a selective high-affinity non-nucleotide antagonist for the P2Y1-R (Nandanan et al., 2000, Boyer et al., 2002). Usefulness of this molecule is significantly extended here by demonstrating methodology for its radiolabeling to high specific radioactivity with tritium and for its application as a radioligand in the first widely useful radioligand binding assay for a P2Y receptor. Interaction of [3H]MRS2279 with a single high-affinity (Kd = 5–10 nM) binding site was observed across a broad range of P2Y1-R–expressing tissues, and the pharmacological selectivity of this binding site was consistent with selective high affinity interaction with the P2Y1-R.
Development of [3H]MRS2279 as a radioligand has followed from our program to synthesize non-nucleotide antagonists for the P2Y1-R. Two major observations have contributed to this success. First, we discovered that bisphosphate analogs of adenine nucleotides were partial agonists or fully competitive antagonists of the P2Y1-R while exhibiting little or no activity at other P2Y receptors (Boyer et al., 1996a). Second, we discovered that the ribose moiety was dispensable in ligand interaction with P2Y1-R (Kim et al., 2000; Nandanan et al., 2000). We followed this observation by generating a series of acyclic-, carbocyclic-, or methanocarba-substitutions of the ribose in the adenine nucleotide backbone of bisphosphate analogs that have retained considerable affinity for the P2Y1-R (Kim et al., 2000, Nandanan et al., 2000, Kim et al., 2001). Some of the most promising of these molecules included methanocarba replacement of the ribose, and MRS2279 exhibits the highest P2Y1-R binding affinity of this series (Boyer et al., 2002). Given that these molecules lack a nucleotide structure, they would not be expected to bind avidly to the thousands of proteins in mammalian genomes that recognize nucleotides. This has been illustrated experimentally with MRS2279, first in experiments showing that this molecule competitively blocks agonist-stimulated activity at the P2Y1-R but not at six other P2Y receptors that are activated by nucleotides (Boyer et al., 2002), and now with [3H]MRS2279, which binds to the recombinant P2Y1-R but not to the P2Y2 or P2Y12 receptor expressed to very high levels in Sf9 insect cells. The low level (<20 fmol/mg protein at concentrations of [3H]MRS2279 that saturate the P2Y1-R) of nonspecific binding of [3H]MRS2279 in membranes prepared from a variety of tissues also illustrates the rather high selectivity of this molecule for the P2Y1-R.
The Ki values determined for a number of P2Y1-R antagonists in competition binding curves with [3H]MRS2279 matched those reported for the KB values of these molecules in analyses of antagonist activity in various P2Y1-R expressing preparations. The most important of these similarities was with [3H]MRS2279 itself, which bound with a Kd that essentially matched the KB values we previously reported for this molecule from Schild analyses measuring competitive antagonism (pKB = 8.10) of 2MeSADP-stimulated inositol phosphate accumulation in 1321N1 human astrocytoma cells stably expressing the human P2Y1-R or competitive antagonism (pKB = 8.05) of 2MeADP-promoted aggregation of human platelets through the P2Y1-R (Boyer et al., 2002). Interestingly, the only tissue in which binding of [3H]MRS2279 to the P2Y1-R was not entirely reliable was turkey erythrocyte membranes. This is ironic given the great value of the turkey erythrocyte P2Y1-R as a model system for developing a variety of useful P2Y1-R agonists and antagonists, including MRS2279 (Boyer et al., 1996a,b, 1998, 2002). The density of P2Y1-R binding sites on the turkey erythrocyte is very low (< 20 fmol/mg) based on our studies with [3H]MRS2279. Our earlier Schild analyses also revealed a somewhat lower Ki (pKB = 7.75) of MRS2279 for the turkey P2Y1-R (Boyer et al., 2002), and perhaps dissociation of bound radioligand from the turkey receptor or the necessity of higher concentrations (and therefore more nonspecific binding) may also contribute to difficulty in detecting P2Y1-R binding with [3H]MRS2279 in turkey erythrocyte membranes. Nonetheless, data obtained with human platelets and rat brain underscore more relevant observations for application of [3H]MRS2279 to study the properties, regulation, and distribution of P2Y1-R in physiologically important mammalian tissues.
The availability of [3H]MRS2279 as a radioligand for the P2Y1-R also makes possible direct determination of the binding affinity of agonists for this receptor. Estimation of binding affinities for agonist molecules by measuring tissue responses is inexact at best, and specific problems are encountered with nucleotides because of their high rates of hydrolysis and interconversion by tissues. Thus, although it is important to define the relative affinities of ADP versus ATP for the P2Y1-R and the agonist versus antagonist action of ATP, these questions have not been unambiguously answered despite much effort by several laboratories (Hechler et al., 1998; Palmer et al., 1998; Simon et al., 2001). The application of [3H]MRS2279 in a radioligand binding assay for the P2Y1-R provides for the first time a means for direct assessment of agonist/receptor interaction but does not fully circumvent problems that have plagued understanding of ADP versus ATP action at the P2Y1-R in the past. That is, incubation with membrane preparations results in nucleotide metabolism; therefore, radioligand binding experiments with [3H]MRS2279 face much the same problems as encountered in tissue or second messenger responses. Indeed, membrane preparations have the added contribution of high activities of intracellular nucleotidases not encountered in studies of P2Y1-R in intact cells. High-level expression of the P2Y1-R in Sf9 insect cells allows assays with small amounts of membrane and therefore reduced hydrolysis of nucleotides. Nonetheless, as illustrated in Fig. 7, significant metabolism and interconversion occurred under the conditions of our assays. Thus, accurate Ki values cannot be calculated from the IC50 values obtained in competition binding curves in our studies. Although we have not taken such an approach, perhaps nonequilibrium measurements of the capacity of ADP versus ATP to inhibit the initial rate of binding of [3H]MRS2279 might reduce these problems. Alternatively, purification of the P2Y1-R to homogeneity will provide conditions in which equilibrium binding assays can be carried out in the assured absence of nucleotide metabolism. Demonstration that [3H]MRS2279 is useful for detecting P2Y1-R in a soluble form after solubilization of membranes with digitonin is encouraging. Moreover, progress has been made in our laboratory in purification of the human P2Y1-R (G. Waldo and T. K. Harden, unpublished observations), and we anticipate determination of Ki values of ADP and ATP and other analogs under conditions with the purified receptor where no metabolism occurs.
The properties of [3H]MRS2279 binding to the recombinant P2Y1-R expressed to high levels in Sf9 cell membranes were largely recapitulated in studies with natively expressed receptor in human platelets or rat brain. Thus, [3H]MRS2279 should prove very useful in studying P2Y1-R distribution and regulation in both human and laboratory animal tissues. The radiostability of the tritium-labeled compound has obvious advantages, but we also are in the process of producing 33P-labeled MRS2279, which should be useful for quantitation of P2Y1-R in tissues in which expression is low. In addition, the aforementioned problems inherent in studying nucleotide agonists will be reduced by the increased specific radioactivity of a 33P-labeled molecule allowing assays with much less tissue and therefore less endogenous nucleotidase activity.
Attempts to label P2Y1-R with radiolabeled agonists have been problematic. We originally proposed that [35S]ADPβS was a useful radioligand for the P2Y1-R on avian erythrocyte membranes (Cooper et al., 1989). Although the selectivity of molecules for inhibition of binding correlated with their activities for activation of phospholipase C through the P2Y-R, we have been unable to obtain unambiguous data proving that the [35S]ADPβS binding site(s) in turkey erythrocyte membranes indeed represented P2Y1-R binding alone. Furthermore, we were unable to convincingly apply this assay to mammalian tissues or preparations expressing native or recombinant P2Y1-R (Harden et al., 1995). Similarly, [35S]deoxyadenosine 5′(α-thio)triphosphate has been proposed to label P2Y1-R and other P2Y receptors in various mammalian tissues and in Cos-7 cells transiently expressing the P2Y1-R (Simon et al., 1995a,b, 1997; Akbar et al., 1996; Webb et al., 1996, 1998). Experiments from our laboratory revealed a very large amount of nonspecific binding of this molecule to membranes in the absence of P2Y1-R expression (Schachter and Harden, 1997), and Motte et al. (1996) concluded that radiolabeled nucleotides were not reliable P2Y receptor radioligands. In addition, the density of binding sites (16–71 pmol/mg protein) reported with deoxyadenosine 5′([α-35S]thio)triphosphate for various guinea pig, rabbit, and rat tissues far exceeds the <1 pmol/mg protein density of binding sites observed for most neurotransmitter and hormone receptors in target tissues. Moreover, the [3H]MRS2279 binding site we have studied here in rat brain membranes occurs at a density of approximately 200 fmol/mg protein, which is similar to the density of many different neurotransmitter receptors in the brain and is greater than 100-fold lower than the density of deoxyadenosine 5′([α-35S]thio)triphosphate binding sites (39 pmol/mg protein) reported for rat brain membranes (Simon et al., 1995a). More recently, Baurand et al. (2001) have 33P-labeled a P2Y1-R antagonist molecule, MRS2179, also previously developed by our group (Boyer et al., 1998). This molecule was shown to bind to intact human platelets with relatively high affinity (Kd = 109 nM). Binding was inhibited by nonradioactive MRS2179 and by adenosine 3′,5′-bisphosphate with Ki values similar to those observed in studies of these two compounds as competitive antagonists of P2Y1-R–promoted responses. Application of [33P]MRS2179 to the study of P2Y1-R in other tissues was not reported. The 5- to 10-fold higher affinity of MRS2279 than MRS2179, as well as the fact that MRS2179 is a true nucleotide analog whereas MRS2279 is not, are two important advantages inherent in the structure of MRS2279.
In summary, we have developed a binding assay for P2Y1-R using a radiolabeled antagonist that has evolved from a series of studies designed to produce high-affinity P2Y1-R selective antagonists. This molecule is applicable for the study of recombinant P2Y1-R in membrane or soluble forms and natively expressed P2Y1-R in human, rat, and mouse tissues. This receptor assay should prove valuable in furthering our understanding of the biological significance and regulation of the P2Y1-R and for further drug development for this therapeutically important target protein.
Acknowledgments
We thank Eduardo Lazarowski for assistance in quantitating ATP and ADP metabolism, Linda Brady in the University of North Carolina Blood Bank for providing out-dated platelets, and David Rinker and Meredith Jones for help in preparation of the manuscript.
This work was supported by Unites States Public Health Service grants GM38213 and HL54889. J.C. was supported by a postdoctoral fellowship from the Pharma Foundation.
ABBREVIATIONS
- P2Y1-R
P2Y1 receptor
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- A3′P5′P
adenosine-3′,5′-bisphosphate
- PPADS
pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid
- PPh3
triphenylphosphine
- DEAD
diethylazidocarboxylate
- THF
tetrahydrofuran
- 2MeSADP
2-methylthio-ADP
- 2MeSATP
2-methylthio-ATP
- MRS2179
N6-methyl-2′-adenosine-3′,5′-bisphosphate
- CHO
Chinese hamster ovary
References
- Akbar GKM, Dasari VR, Webb TE, Ayyanathan K, Pillarisetti K, Sandhu AK, Athwal RS, Daniel JL, Ashby B, Barnard EA, et al. Molecular cloning of a novel P2 purinoceptor from human erythroleukemia cells. J Biol Chem. 1996;271:18363–18367. doi: 10.1074/jbc.271.31.18363. [DOI] [PubMed] [Google Scholar]
- Alvarado-Castillo C, Lozano-Zahain P, Mateo J, Harden TK, Boyer JL. A fusion protein of the human P2Y1 receptor and NTPDase1 exhibits functional activities of the native receptor and ectoenzyme and reduced signaling responses to endogenously released nucleotides. Mol Pharmacol. 2002;62:521–528. doi: 10.1124/mol.62.3.521. [DOI] [PubMed] [Google Scholar]
- Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′, 5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′, 5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Romero T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996a;50:1323–1329. [PubMed] [Google Scholar]
- Boyer JL, Schachter JB, Sromek SM, Palmer RK, Jacobson KA, Nicholas RA, Harden TK. Avian and human homologues of the P2Y1 receptor: pharmacological, signaling and molecular properties. Drug Dev Res. 1996b;39:253–261. doi: 10.1002/(sici)1098-2299(199611/12)39:3/4<253::aid-ddr4>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Zohn IE, Jacobson KA, Harden TK. Differential effects of putative P2-purinergic receptor antagonists on adenylyl cyclase- and phospholipase C-coupled P2Y purinergic receptors. Br J Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine- bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CL, Morris AJ, Harden TK. Guanine nucleotide-sensitive interaction of a radiolabeled agonist with a phospholipase C-linked P2-purinergic receptor. J Biol Chem. 1989;264:6202–6206. [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Harden TK, Barnard EA, Boeynaems JM, Burnstock G, Dubyak GR, Hourani SMO, Insel PA. P2Y receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. London: IUPHAR Media; 1998a. pp. 209–217. [Google Scholar]
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995;35:541–579. doi: 10.1146/annurev.pa.35.040195.002545. [DOI] [PubMed] [Google Scholar]
- Harden TK, Lazarowski ER, Boucher RC. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- Harden TK, Nicholas RA, Schachter JB, Lazarowski ER, Boyer JL. Pharmacological selectivities of molecularly defined subtypes of P2Y receptors. In: Turner JT, Weisman GA, Fedan JS, editors. The P2 Nucleotide Receptors. Totowa, NJ: Humana Press; 1998b. pp. 109–134. [Google Scholar]
- Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53:727–733. [PubMed] [Google Scholar]
- Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Barnard EA, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. P2X receptors. In: Girdlestone D, editor. The IUPHAR Compendium of Receptor Characterization and Classification. London: IUPHAR Media; 2000. pp. 291–305. [Google Scholar]
- Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: structure-activity relationships and receptor docking. J Med Chem. 2001;44:3092–3108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Gallo-Rodriguez C, Jang S-Y, Nandanan E, Adams M, Harden TK, Boyer JL, Jacobson KA. Acyclic analogues of deoxyadenosine 3′, 5′-bisphosphates as P2Y1 receptor antagonists. J Med Chem. 2000;42:746–755. doi: 10.1021/jm9905211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte S, Swillens S, Boeynaems JM. Evidence that most high-affinity ATP binding sites on aortic endothelial cells and membranes do not correspond to P2 receptors. Eur J Pharmacol. 1996;307:201–209. doi: 10.1016/0014-2999(96)00234-8. [DOI] [PubMed] [Google Scholar]
- Nandanan E, Camaioni E, Jang SY, Kim YC, Cristalli G, Herdewijn P, Secrist JA, 3rd, Tiwari KN, Mohanram A, Harden TK, et al. Structure-activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, et al. Synthesis, biological activity and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Perich JW, Johns RB. Di-tert-butyl N,N-niethylphosphoramidite. A new phosphorylating agent for the efficient phosphorylation of alcohols. Synthesis. 1988;2:142–144. [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Schachter JB, Harden TK. An examination of deoxyadenosine 5′(α-thio)triphosphate as a ligand to define P2Y receptors and its selectivity as a low potency partial agonist of the P2Y1 receptor. Br J Pharmacol. 1997;121:338–344. doi: 10.1038/sj.bjp.0701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1 receptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Vigne P, Eklund KM, Michel AD, Carruthers AM, Humphrey PP, Frelin C, Barnard EA. Activity of adenosine diphosphates and triphosphates on a P2Y(T)-type receptor in brain capillary endothelial cells. Br J Pharmacol. 2001;132:173–182. doi: 10.1038/sj.bjp.0703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Webb TE, Barnard EA. Characterization of a P2Y purinoceptor in the brain. Pharmacol Toxicol. 1995a;76:302–307. doi: 10.1111/j.1600-0773.1995.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Simon J, Webb TE, Barnard EA. Distribution of [35S]dATPαS binding sites in the adult rat neuraxis. Neuropharmacology. 1997;36:1243–1251. doi: 10.1016/s0028-3908(97)00124-x. [DOI] [PubMed] [Google Scholar]
- Simon J, Webb TE, King BF, Burnstock G, Barnard EA. Characterization of a recombinant P2Y purinoceptor. Eur J Pharmacol. 1995b;291:281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Webb TE, Kaplan MG, Barnard EA. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun. 1996;219:105–110. doi: 10.1006/bbrc.1996.0189. [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Barnard EA. Regional Distribution of [35S]2′-Deoxy 5′-O-(1-thio) ATP binding sites and the P2Y1 messenger RNA within the chick brain. Neuroscience. 1998;84:825–837. doi: 10.1016/s0306-4522(97)00478-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]