Figure 1.
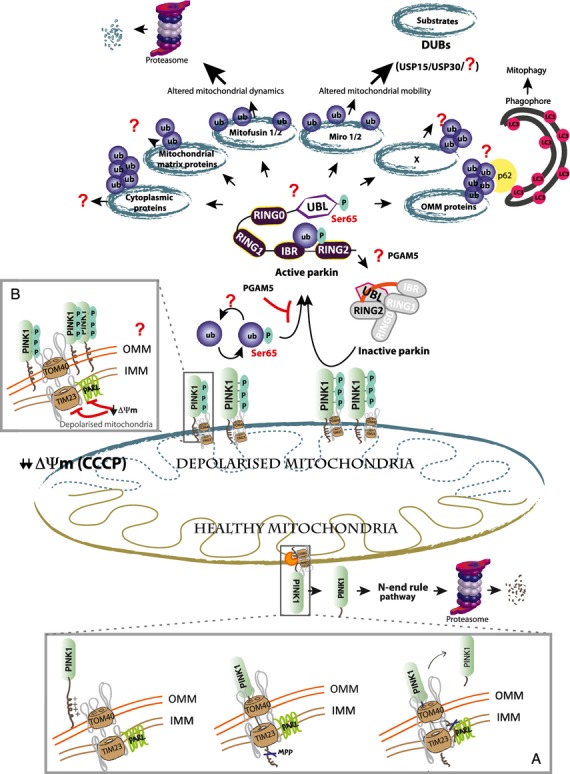
PINK1-Parkin signalling. Under healthy conditions, PINK1 is imported to the mitochondria via a multisubunit complex, including TOM40 on the outer mitochondrial membrane (OMM) and TIM23 in the inner mitochondrial membrane (IMM). Upon entry, PINK1 is sequentially cleaved by proteases MPP and PARL between residues 103 and 104. The resulting C-terminal fragment is rapidly degraded via the N-end rule pathway (inset A). Upon mitochondrial depolarization (e.g. induced by uncouplers), mitochondrial import and cleavage by PARL is inhibited, resulting in PINK1 stabilization and accumulation at the OMM, which in turn leads to PINK1 autophosphorylation and activation (inset B). Parkin exists in an inactive state mediated by multiple autoinhibitory surface interactions. Upon activation, PINK1 phosphorylates Parkin at Ser65 within its Ubl domain and ubiquitin at Ser65. Phosphorylation of Parkin Ubl Ser65 and binding of ubiquitin Ser65 confers maximal activation of Parkin E3 ubiquitin ligase activity. Multiple Parkin substrates have been identified, implicating its role in distinct mitochondrial signalling processes, although only a few substrates have been characterized in detail. Parkin-dependent ubiquitylated OMM substrates interact with ubiquitin binding domain-containing proteins (UBD) e.g. p62 that stimulate recruitment of autophagy machinery to induce mitophagy. Targeting of mitochondrial GTPases Miro1 and Mfn1/2 may influence mitochondrial transport and dynamics, respectively. The negative regulators of this pathway remain largely unknown, although recent work has suggested roles for USP15 and USP30 as deubiquitylases.
